Abstract
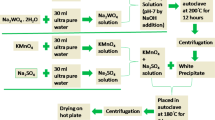
ZnMn2O4 (ZMO) nanopowders have been synthesized by a hydrothermal method using different surfactants [cetyltrimethylammonium bromide (CTAB), polyethylene glycol (PEG)-400, and Polysorbate-80]. The as-prepared ZnMn2O4 samples exhibited single phase with tetragonal structure, showing honeycomb, spinel microsphere, and flower-cluster morphology, respectively. Cyclic voltammetry curves for all samples presented rectangular shape with symmetric nature and good cycling properties, with no obvious redox peak. Galvanostatic charge–discharge curves were triangular and symmetric. The specific capacitance of the ZnMn2O4 nanopowders gradually decreased with increase of the scanning rate. ZMO-PEG exhibited higher specific capacitance of 191 F g−1 at scan rate of 5 mV s−1 and retained superior large-current cycling stability of 98.4% after 1000 cycles compared with ZMO-CTAB (93.8%) or ZMO-Polysorbate-80 (97.7%). Electrochemical impedance spectroscopy revealed that the ZnMn2O4 nanopowders had low resistance. These results suggest that ZnMn2O4 nanopowders have good capacitance characteristics.
Similar content being viewed by others
References
Y. Li, L.-B. Kong, M.-C. Liu, W.-B. Zhang, and L. Kang, Mater. Lett. 186, 289 (2017).
H. Wu, G. Wu, Y. Ren, X. Li, and L. Wang, Chemistry 22, 8864 (2016).
G.R. Xu, X.P. Min, Q.L. Chen, Y. Wen, A.P. Tang, and H.S. Song, J. Alloys Compd. 691, 1018 (2017).
T. Zhang, L.-B. Kong, M.-C. Liu, Y.-H. Dai, K. Yan, B. Hu, Y.-C. Luo, and L. Kang, Mater. Des. 112, 88 (2016).
W. Ma, S. Chen, S. Yang, W. Chen, Y. Cheng, Y. Guo, S. Peng, S. Ramakrishna, and M. Zhu, J. Power Sources 306, 481 (2016).
M. Huang, Y. Zhang, F. Li, L. Zhang, Z. Wen, and Q. Liu, J. Power Sources 252, 98 (2014).
H. Wu, G. Wu, Y. Ren, L. Yang, L. Wang, and X. Li, J. Mater. Chem. C 3, 7677 (2015).
G.H. Yue, Y.C. Zhao, C.G. Wang, X.X. Zhang, X.Q. Zhang, and Q.S. Xie, Electrochim. Acta 152, 315 (2015).
H. Wu, G. Wu, and L. Wang, Powder Technol. 269, 443 (2015).
T. Zhang, Y. Gao, H. Yue, H. Qiu, Z. Guo, Y. Wei, C. Wang, G. Chen, and D. Zhang, Electrochim. Acta 198, 84 (2016).
L.X. Zhang, Y.L. Wang, H.F. Jiu, H.Y. Qiu, and H.Y. Wang, Ceram. Int. 41, 9655 (2015).
D. Cai, D. Wang, H. Huang, X. Duan, B. Liu, L. Wang, Y. Liu, Q. Li, and T. Wang, J. Mater. Chem. A 3, 11430 (2015).
L. Xiao, Y. Yang, J. Yin, Q. Li, and L. Zhang, J. Power Sources 194, 1089 (2009).
X.X. Lin, Y.F. Zhu, and W.Z. Shen, J. Phys. Chem. C 113, 1812 (2009).
Z. Wang, J. Zhang, Y. Yang, and Ling, J. Wuhan Univ. Technol. (Mater. Sci. Edn.) 30, 1159 (2015).
P. Li, J. Liu, Y. Liu, Y. Wang, Z. Li, W. Wu, Y. Wang, L. Yin, H. Xie, and M. Wu, Electrochim. Acta 180, 164 (2015).
W. Dang, F. Wang, Y. Ding, C. Feng, and Z. Guo, J. Alloys Compd. 690, 72 (2017).
F.M. Courtel, Y. Abu-Lebdeh, and I.J. Davidson, Electrochim. Acta 71, 123 (2012).
F. Courtel, H. Duncan, Y. Abulebdeh, and I. Davidson, J. Mater. Chem. 21, 10206 (2011).
L. Zhao, X. Li, and J. Zhao, Appl. Surf. Sci. 268, 274 (2013).
A. Sahoo and Y. Sharma, Mater. Chem. Phys. 149, 721 (2015).
R. Gherbi, Y. Bessekhouad, and M. Trari, J. Phys. Chem. Solids 89, 69(9) (2016).
B. Ameri, S.S.H. Davarani, H.R. Moazami, and H. Darjazi, J. Alloys Compd. 720, 5 (2017).
N. Guo, X.Q. Wei, X.L. Deng, and X.J. Xu, Appl. Surf. Sci. 356, 1127 (2015).
L. Demarconnay, E. Raymundo-Piñero, and F. Béguin, Electrochem. Commun. 1275, 12 (2010).
C. Portet, P.L. Taberna, and P. Simon, Electrochim. Acta 4174, 50 (2005).
G. Zhou, J. Zhu, and Y. Chen, Electrochim. Acta 450, 123 (2014).
K. Qiu, Y. Lu, and D. Zhang, Mesoporous Nano Energy 687, 11 (2015).
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 51261015) and the Natural Science Foundation of Gansu Province, China (No. 1308RJZA238).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Wei, Z., Zhao, W. et al. Microstructure and Electrochemical Properties of ZnMn2O4 Nanopowder Synthesized Using Different Surfactants. J. Electron. Mater. 47, 6428–6436 (2018). https://doi.org/10.1007/s11664-018-6544-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6544-7