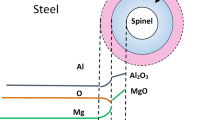
Abstract
Calcium treatment of steel is typically employed to modify alumina inclusions to liquid calcium aluminates. However, injected calcium also reacts with the dissolved sulfur to form calcium sulfide. The current work aims to develop a kinetic model for the evolution of oxide and sulfide inclusions in Al-killed alloyed steel during Ca treatment in the ladle refining process. The model considers dissolution of the calcium from the calcium bubbles into the steel and reduction of calcium oxide in the slag to dissolved calcium. A steel–inclusion kinetic model is used for mass transfer to the inclusion interface and diffusion within the calcium aluminate phases formed on the inclusion. The inclusion–steel kinetic model is then coupled with a previously developed steel–slag kinetic model. The coupled inclusion–steel–slag kinetic model is applied to the chemical composition changes in molten steel, slag, and evolution of inclusions in the ladle. The result of calculations is found to agree well with an industrial heat for species in the steel as well as inclusions during Ca treatment.










Similar content being viewed by others
References
K.G. Rackers and B.G. Thomas: 78th Steelmak. Conf. Proc., Iron and Steel Society, Nashville, 1995, pp. 723–34.
[2] S. W. Robinson, I. W. Martin, and F. B. Pickering: Met. Technol., 1979, vol. 6, pp. 157–69.
L. Zhang and B.G. Thomas: XXIV Natl Steelmak. Symp., Morelia, Mich, Mexico, 2003, pp. 138–83.
[4] E.T. Turkdogan: Fundamentals of Steelmaking, 1st Ed., Maney, London, 2010, pp. 285–90.
[5] A. Ghosh: Secondary Steelmaking: Principles and Applications, 1st Ed., CRC Press, London, 2001, pp. 203–17.
[6] S. Basak, R. Kumar Dhal, and G. G. Roy: Ironmak. Steelmak., 2010, vol. 37, pp. 161–68.
[7] D. Lu, G. A. Irons, and W. Lu: Ironmak. Steelmak., 1994, vol. 21, pp. 362–72.
[8] Y. Higuchi, M. Numata, Sh. Fukagawa, and K. Shinme: ISIJ Int., 1996, vol. 36, pp. 151–54.
[9] Y. Ito, M. Suda, Y. Kato, H. Nakato, and K. Sorimachi: ISIJ Int., 1996, vol. 36, pp. S148–50.
H. Visser, R. Boom, and M. Biglari: ATS Int. Steelmak. Conf., 2008, pp. 172–80.
[11] Z. Han, L. Liua, M. Lind, and L. Holappa: Acta Metall. Sin., 2006, vol. 19, pp. 1–8.
[12] Y. Tabatabaei, K. S. Coley, G. A. Irons, and S. Stanley: Metall. Mater. Trans. B, 2017, vol. 46, pp. 2820–25.
K.J. Graham and G.A. Irons: in Int. Symp. Highly Innov. Nov. Oper. “Future Steelmak. Metall., 2010, pp. 65–74.
A. Galindo, G.A. Irons, K.S. Coley, and S. Sun: in Challenges Transform. Solut. Sustain. Steelmak. Cast. Environ. Metall. Innov. CTSSC-EMI Symp. 2015, Tokyo, Japan, 2015.
[15] A. Harada, N. Maruoka, H. Shibata, and Sh. Kitamura: ISIJ Int., 2013, vol. 53, pp. 2110–17.
[16] A. Harada, N. Maruoka, H. Shibata, M. Zeze, and N. Asahara: ISIJ Int., 2014, vol. 54, pp. 2569–77.
D. Kumar and P.C. Pistorius: Proc. 10th Int. Conf. Molten Slags, Fluxes Salts, Wiley, Hoboken, NJ, USA, 2016, pp. 145–53.
[18] J. H. Shin, Y. Chung, and J. H. Park: Metall. Mater. Trans. B, 2017, vol. 48, pp. 46–59.
K. Graham: PhD Thesis, McMaster University, 2008, pp. 172–81.
D.-Z. Lu: PhD Thesis, McMaster University, 1992, pp. 206–29.
[21] O. Levenspiel: Chemical Reaction Engineering, 2nd Ed., John Wiley & Sons, New York, 1999, pp. 570–577.
[22] N. Verma, P. C. Pistorius, R.J. Fruehan, M.S. Potter, H.G. Oltmann, and E.B. Pretorius: Metall. Mater. Trans. B, 2012, vol. 43, pp. 830–40.
N. Verma, P.C. Pistorius, R.J. Fruehan, and R.J. Lee: in Mater. Sci. Technol. Conf. Exhib. 2009, MS T’09, Pittsburgh, PA, 2009, pp. 1042–53.
[24] S. F. Yang, J. Sh. Li, Z. F. Wang, J. Li, and L. Lin: Int. J. Miner. Metall. Mater., 2011, vol. 18, pp. 18–23.
[25] J. Szekely and N. J. Themelis: Rate Phenomena in Process Metallurgy, 1st Ed., Wiley-Interscience, New York, 1971.
[26] F. Oeters: Metallurgy of Steelmaking, 1st Ed., Düsseldorf: Verlag Stahleisen, 1994.
[27] C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, and K. Hack: Calphad, 2002, vol. 26, pp. 189–228.
[28] C.W. Bale, E. Bélisle, P. Chartrand, S. A. Decterov, G. Eriksson, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, C. Robelin, and S. Petersen: Calphad, 2009, vol. 33, pp. 295–311.
[29] M. Hino and K. Ito: Thermodynamic Data for Steelmaking, 1st Ed., Tohoku University Press, Tokyo, 2010, pp. 16–17.
[30] H. Fujiwara, A. Hattori, and E. Ichise: Tetsu-To-Hagane/Journal Iron Steel Inst. Japan, 1999, vol. 85, pp. 201–7.
[31] Q. Han, X. Zhang, D. Chen, and P. Wang: Metall. Mater. Trans. B, 1988, vol. 19B, pp. 617–22.
[32] D. G. C. Robertson, B. Deo, and S. Ohguchi: Ironmak. Steelmak., 1984, vol. 11, pp. 41–55.
M. L. Kapoor and M. G. Frohberg: in Chem. Metall. Iron Steel, 1971, pp. 17–22.
H. Gaye and J. Welfringer: in Second Int. Symp. Metall. Slags Fluxes, 1984, pp. 357–75.
F. Schamber: Introduction to Automated Particle Analysis by Focused Electron Beam, 2009.
[36] R. Higginson and C.M. Sellars: Worked Examples in Quantitative Metallography, 1st Ed., Maney, London, 2003, pp. 68–76.
[37] P. Kaushik, H. Pielet, and H. Yin: Iron Steel Technol., 2009, vol. 6, pp. 82–99.
[38] N. Verma, P. C. Pistorius, R.J Fruehan, M. Potter, M. Lind, and S. Story: Metall. Mater. Trans. B, 2011, vol. 42, pp. 711–19.
[39] M. Nuspl, W. Wegscheider, J. Angeli, W. Posch, and M. Mayr: Anal. Bioanal. Chem., 2004, vol. 379, pp. 640–45.
[40] B. G. Bartosiaki, J. A. M. Pereira, W. V. Bielefeldt, and A. C. F. Vilela: J. Mater. Res. Technol., 2015, vol. 4, pp. 235–40.
[41] J. H. Shin and J. H. Park: Metall. Mater. Trans. B, 2017, vol. 48, pp. 2820–25.
S. Sun, S. Waterfall, N. Strobl, D. Liao, and D. Holdridge: in 8th Int. Symp. High-Temperature Metall. Process., 2017, pp. 347–57.
[43] H.A. Schwartz: Met. Alloy., 1934, vol. 5, pp. 139–40.
Acknowledgments
The authors are grateful for the support of the Members of the McMaster Steel Research Centre and the Natural Sciences and Engineering Research Council of Canada. The strong support and collaboration with ArcelorMittal Dofasco in planning and executing the work was vital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 1, 2017.
Appendix: Volumetric Size Distributions of Inclusions
Appendix: Volumetric Size Distributions of Inclusions
An automated inclusion analysis SEM technique, ASPEX, was used to obtain planar distribution of inclusion on the polished section. Particle sizes are divided into number of size groups after they are measured on plane sections. The number and size of particles in the plane section is determined, but to obtain the size distribution on a volume basis, the analysis of the obtained data is required. It is assumed that the particles are spherical so that equivalent sphere diameters or equivalent circle diameters can be considered. ASPEX measures equivalent circle diameters of inclusions which can be presented in a particle size distribution histogram.
The number of circles per unit area NA as a result of NV spheres per unit volume of diameter D is
So, larger spheres are more likely to be intersected by the plane of the polished section. Moreover, a sphere may be sectioned anywhere in its diameter. But, only largest spheres can lead to largest circle diameter at the sectioning surface. Therefore, the probability of spotting circles in this largest size group could be calculated and the residual probability distributed to the smaller size groups of circles. Then, circles from the next smallest size group of spheres are calculated and so on. Using this approach, the size distribution of spheres in the volume could be derived from the measurement of the size distributions of circles on the sectioning plane. The details of the method can be found in Reference 42. Schwartz[36] and Saltykov[43] developed a matrix of coefficients (α(i, j)) for the number of circles in size group (i) arising from spheres in size group (j) using probability distributions for sectioning randomly distributed spheres of sizes in k equal size groups. The number of spheres per unit volume in size group j from the numbers of circles in size groups i is
where j = 1 to k, Δ is the size interval used in the histograms and k is the number of size groups.
Rights and permissions
About this article
Cite this article
Tabatabaei, Y., Coley, K.S., Irons, G.A. et al. Model of Inclusion Evolution During Calcium Treatment in the Ladle Furnace. Metall Mater Trans B 49, 2022–2037 (2018). https://doi.org/10.1007/s11663-018-1266-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1266-z