Abstract
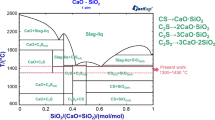
The phosphate-enrichment behavior has experimentally been investigated in CaO-SiO2-FeO-Fe2O3-P2O5 steelmaking slags. The reaction ability of structural units in the slags has been represented the mass action concentration \( N_{i} \) from the developed ion and molecule coexistence theory (IMCT)-\( N_{i} \) model based on the IMCT. The defined enrichment possibility \( N_{{{\text{c}}i{\text{ {-}c}}j}} \) and enrichment degree \( R_{{{\text{c}}i{\text{{-}c}}j}} \) of solid solutions containing P2O5 from the developed IMCT-\( N_{i} \) model have been verified from the experimental results. The effects of binary basicity, the mass percentage ratio \( {{ ( {\text{pct Fe}}_{t} {\text{O)}}} \mathord{\left/ {\vphantom {{ ( {\text{pct Fe}}_{t} {\text{O)}}} { ( {\text{pct CaO)}}}}} \right. \kern-0pt} { ( {\text{pct CaO)}}}} \), and mass percentage of P2O5 in the initial slags on phosphate-enrichment behavior in the slags has also been discussed. The results show that the P2O5 component can easily be bonded by CaO to form tricalcium phosphate 3 CaO·P2O5, and the formed 3CaO·P2O5 can react with the produced dicalcium silicate 2CaO·SiO2 to generate solid-solution 2CaO·SiO2-3CaO·P2O5 under fixed cooling conditions. The maximum value of the defined enrichment degree \( R_{{{\text{C}}_{ 2} {\text{S{-}}} {\text{C}}_{ 3} {\text{P}}}} \) of solid-solution 2CaO·SiO2-3CaO·P2O5 is obtained as 0.844 under conditions of binary basicity as 2.5 and the mass percentage ratio \( {{ ( {\text{pct Fe}}_{t} {\text{O)}}} \mathord{\left/ {\vphantom {{ ( {\text{pct Fe}}_{t} {\text{O)}}} { ( {\text{pct CaO)}}}}} \right. \kern-0pt} { ( {\text{pct CaO)}}}} \) as 0.955 at fixed cooling conditions.














Similar content being viewed by others
Abbreviations
- \( a_{{{\text{R,}}i}} \) :
-
Activity of components i in slags relative to pure solid or liquid component i as standard state with mole fraction \( x_{i} \) as concentration unit and following the Raoult’s law, i.e., \( a_{{{\text{R,}}i}} = x_{i} \gamma_{i} \), (−)
- \( b_{i} \) :
-
Mole number of component i in 100-g slags before reaction equilibrium, having the same meaning with \( n_{i}^{0} \), (mol)
- \( \Delta_{\text{r}} G_{{{\text{m, }}i}}^{\Theta } \) :
-
Standard molar Gibbs free energy change of reaction for forming component i or structural unit i, (J/mol)
- \( K_{i}^{\Theta } \) :
-
Standard equilibrium constant of chemical reaction for forming component i or structural unit i, (−)
- M i :
-
Relative atomic mass of element i or relative molecular mass of component i, (−)
- \( n_{i} \) :
-
Equilibrium mole number of structural unit i or ion couple i in 100-g slags based on the IMCT, (mol)
- \( \Sigma n_{i} \) :
-
Total equilibrium mole number of all structural units in 100-g slags based on the IMCT, (mol)
- \( N_{i} \) :
-
Mass action concentration of structural unit i or ion couple i in slags based on the IMCT, (−)
- \( N_{{{\text{c}}i {\text{{-}c}}j}} \) :
-
Defined enrichment possibility of solid solution containing P2O5 based on the calculated mass action concentration \( N_{i} \) of complex molecule ci and cj, (−)
- R :
-
Gas constant, (8.314 J/(mol·K))
- \( R_{{{\text{c}}i {\text{{-}c}}j}} \) :
-
Defined enrichment degree of solid solution containing P2O5, (−)
- \( R_{\text{P}} \) :
-
Determined enrichment ratio \( R_{\text{P}} \) of phosphorus, (−)
- T :
-
Absolute temperature, (K)
- (pct i):
-
Mass percentage of component i in slags, (−)
- (i):
-
Species i in slag phase, (−)
- \( \gamma_{i} \) :
-
Activity coefficient of component i in slags related with activity \( a_{{{\text{R,}}i}} \), (−)
- ci :
-
Molecule ci or compound ci or compound i of calcium silicates, (−)
- cj :
-
Molecule cj or compound cj or compound j containing P2O5, (−)
References
H. Ono, A. Inagaki, T. Masui, H. Narita, T. Mitsuo, S. Nosaka, and S. Gohda: Tetsu-to-Hagané, 1980, vol. 66, no. 9, pp. 1317-23.
K. Morita, M.X. Guo, N. Oka, and N. Sano: J. Mater. Cycles Waste Manag., 2002, vol. 4, no. 2, pp. 93-101.
S. Takeuchi, N. Sano, and Y. Matsushita: Tetsu-to-Hagané, 1980, vol. 66, no. 14, pp. 2050-7.
Z.T. Sui and P.X. Zhang: ACTA Metall. Sin, 1997, vol. 33, no. 9, pp. 943-51.
X.R. Wu, L.S. Li, and Y.C. Dong: ISIJ Int., 2007, vol. 47, no. 3, pp. 402-7.
L. Zhang, L.N. Zhang, M.Y. Wang, G.Q. Li, and Z.T. Sui: ISIJ Int., 2006, vol. 46, no. 3, pp. 458-65.
W. Fix, H. Heyman, and R. Heinke: J. Am. Ceram. Soc., 1969, vol. 52, no. 6, pp. 346-47.
K. Ito, M. Yanagisawa, and N. Sano: Tetsu-to-Hagané, 1982, vol. 68, no. 2, pp. 342-4.
R. Inoue and H Suito: ISIJ Int., 2006, vol. 46, no. 2, pp. 174-9.
H Suito and R. Inoue: ISIJ Int., 2006, vol. 46, no. 2, pp. 180-7.
R. Inoue and H Suito: ISIJ Int., 2006, vol. 46, no. 2, pp. 188-94.
T. Hamano, S. Fukagai, and F. Tsukihashi: ISIJ Int., 2006, vol. 46, no. 4, pp. 490-5.
S. Fukagai, T. Hamano, and F. Tsukihashi: ISIJ Int., 2007, vol. 47, no. 1, pp. 187-9.
X. Yang, H. Matsuura, and F Tsukihashi: ISIJ Int., 2009, vol. 49, no. 4, pp. 1298-307.
X. Yang, H. Matsuura, and F. Tsukihashi: Tetsu-to-Hagané, 2009, vol. 95, no. 3, pp. 268-74.
X. Yang, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2010, vol. 50, no. 5, pp. 702-11.
F. Pahlevani, S. Kitamura, H. Shibata, and N. Maruoka: ISIJ Int., 2010, vol. 50, no. 6, pp. 822-9.
H. Kubo, K. Matsubae-yokoyama, and T. Nagasaka: ISIJ Int., 2010, vol. 50, no. 1, pp. 59-64.
K. Matsubae-Yokoyama, H. Kubo, and T. Nagasaka: ISIJ Int., 2010, vol. 50, no. 1, pp. 65-70.
X.M. Yang, M. Zhang, J.L. Zhang, P.C. Li, J.Y. Li, and J. Zhang: Steel Res. Int., 2014, vol. 85, no. 3, pp. 347-75.
J. Björkvall, D. Sichen, and S. Seetharaman: High Temp. Mater. Processes, 1999, vol. 18, no. 4, pp. 253-68.
S. Basu, A. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B, 2008, vol. 39B, no. 3, pp. 447-56.
J. Bygdén, D. Sichen, and S. Seetharaman: Steel Res., 1994, vol. 65, no. 10, pp. 421-8.
P. Fredriksson and S. Seetharaman: Steel Res. Int., 2004, vol. 75, no. 4, pp. 240-6.
P. Fredriksson and S. Seetharaman: Steel Res. Int., 2004, vol. 75, no. 6, pp. 357-65.
J. Björkvall, D. Sichen, V. Stolyarova, and S. Seetharaman: Glass Phys. Chem., 2001, vol. 27, no. 2, pp. 132-47.
C. Borgianni and P. Granati: Metall. Trans. B, 1977, vol. 8, no. 1, pp. 147-51.
J.D. Sommerville, I. Ivanchev, and H.B. Bell: Proc. Int. Symp. on Metallurgical Chemistry—Applications in Ferrous Metallurgy, The Iron and Steel Institute, London, U.K., 1973, pp. 23–25.
G. Ottonello: J. Non-Cryst. Solids, 2001, vol. 282, no. 1, pp. 72-85.
D.P. Tao: Metall. Mater. Trans. B, 2006, vol. 37B, no. 6, pp. 1091-7.
L. Zhang, S. Sun, and S. Jahanshahi: J. Phase Equilib. Diff., 2007, vol. 28, no. 1, pp. 121-9.
M. Modigell, A. Traebert, P. Monheim, S. Petersen, and U. Pickartz: Comput. Chem. Eng., 2001, vol. 25, nos. 4-6, pp. 723-7.
M.L. Kapoor and M.G. Frohberg: Theoretical Treatment of Activities in Silicate Melts, Chemical Metallurgy of Iron and Steel, The Iron and Steel Institute, London, U.K., 1971, pp. 17-22.
A.T. Dinsdale, J.A. Gisby, T.I. Barry, A. Gibbon, and A.L. Davies: Proceedings of ‘Pyrometallurgy ‘95’, The Institute of Mining and Metallurgy, Cambridge, U.K., 1995.
C. Chen and S. Jahanshah: Metall. Mater. Trans. B, 2010, vol. 41B, no. 6, pp. 1166-74.
A.D. Pelton, S.A. Degterov, G. Eriksson, C. Robelin, and Y. Dessureault: Metall. Mater. Trans. B, 2000, vol. 31B, no. 4, pp. 651-9.
A.D. Pelton and P. Chartrand: Metall. Mater. Trans. A, 2001, vol. 32A, no. 6, pp. 1355-60.
P. Chartrand and A.D. Pelton: Metall. Mater. Trans. A, 2001, vol. 32A, no. 6, pp. 1397-407.
A. Pelton, P. Chartrand, and G. Eriksson: Metall. Mater. Trans. A, 2001, vol. 32A, no. 6, pp. 1409-16
Y.B. Kang and A.D. Pelton: Metall. Mater. Trans. B, 2009, vol. 40B, no. 6, pp. 979-94
I.H. Jung, Y.B. Kang, S.A. Decterov, and A.D. Pelton: Metall. Mater. Trans. B, 2004, vol. 35B, no. 2, pp. 259-68.
G Eriksson and A.D. Pelton: Metall. Trans. B, 1993, vol. 24, no. 5, pp. 795-805.
A. Kondratiev and E. Jak: Metall. Mater. Trans. B, 2005, vol. 36B, no. 5, pp. 623-38.
A.D. Pelton and M. Blander: in Second International Symposium on Metallurgical Slags and Fluxes, TMS-AIME, Lake Tahoe, NV, 1984.
S. Ban-ya: ISIJ Int., 1993, vol. 33, no. 2, pp. 2-11.
B. Hallstedt, M. Hillert, M. Selleby, and B. Sundman: CALPHAD, 1994, vol. 18, no. 1, pp. 31-7.
E. Tijskens, W.A. Viaene, and P. Geerlings: Phys. Chem. Miner., 1995, vol. 22, no. 3, pp. 186-99.
L.C. Oertel and A. Costa e Silva:CALPHAD, 1999, vol. 23(3–4), pp. 379–91.
X.M. Yang, J.P. Duan, C.B. Shi, M. Zhang, Y.L Zhang, and J.C. Wang: A Metall. Mater. Trans. B, 2011, vol. 42B, no. 4, pp. 738-70.
X.M. Yang, C.B. Shi, M. Zhang, J.P. Duan, and J. Zhang: Metall. Mater. Trans. B, 2011, vol. 42B, no. 5, pp. 951-76.
X.M. Yang, C.B. Shi, M. Zhang, and J. Zhang: Steel Res. Int., 2012, vol. 83, no. 3, pp. 244-58.
J. Zhang: Computational Thermodynamics of Metallurgical Melts and Solutions, Metallurgical Industry Press, Beijing, China, 2007.
Verein Deutscher Eisenhüttenleute: Slag Atlas, 2nd ed., Woodhead Publishing Limited, Cambridge, U.K., 1995.
J.X. Chen: Handbook of Common Figures, Tables and Data for Steelmaking, Metallurgical Industry Press, Beijing, China, 1984.
C. Nassaralla and R.J. Fruehan: Metall. Trans. B, 1992, vol. 23B, no. 2, pp. 117-23.
M. Timucin and A. Muan: J. Am. Ceram. Soc., 1992, vol. 75, no. 6, pp. 1399-406.
I. Barin, O. Knacke, and O. Kubaschewski: Thermochemical Properties of Inorganic Substances, Supplement. Springer, New York, NY, 1977, p. 392.
E.T. Turkdogan. Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, pp. 8-12.
K. Narita and K. Shinji: Kobe Steel Eng. Reports, 1969, vol. 19, pp. 25-42.
The Japan Society for the Promotion of Science: The 19th Committee on Steelmaking. Steelmaking Data Sourcebook, Gordon and Breach Science Publishers, New York, NY, 1988.
H. Suito, A. Ishizaka, R. Inoue, and Y. Takahashi: Tetsu-to-Hagané, 1979, vol. 65, no. 13, pp. 1848-57.
E.T. Turkdogan: ISIJ Int., 2000, vol. 40, no. 10, pp. 964-70.
R. Nagabayashi, M. Hino, and S. Ban-ya: Tetsu-to-Hagané, 1988, vol. 74, no. 9, pp. 1770-7.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (Nos. 51372019, 51174186, 51072022, and 50874013) and the National Basic Research Program of China (No. 2014CB643401).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 20, 2013.
Rights and permissions
About this article
Cite this article
Li, Jy., Zhang, M., Guo, M. et al. Enrichment Mechanism of Phosphate in CaO-SiO2-FeO-Fe2O3-P2O5 Steelmaking Slags. Metall Mater Trans B 45, 1666–1682 (2014). https://doi.org/10.1007/s11663-014-0085-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0085-0