Abstract
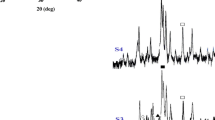
The effects of process parameters on the crystallization and morphology of hydroxyapatite (Ca10(PO4)6(OH)2, HA) powders synthesized from dicalcium phosphate dihydrate (CaHPO4·2H2O, DCPD) using a hydrolysis method have been investigated. X-ray diffraction (XRD), Fourier-transform infrared (FT-IR) spectra, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and selected area electron diffraction (SAED) were used to characterize the synthesized powders. When DCPD underwent hydrolysis in 2.5 NaOH solution (Na(aq)) at 303 K to 348 K (30 °C to 75 °C) for 1 hour, the XRD results revealed that HA was obtained for all the as-dried samples. The SEM morphology of the HA powders for DCPD hydrolysis produced at 348 K (75 °C) shows regular alignment and a short rod shape with a size of 200 nm in length and 50 nm in width. With DCPD hydrolysis in 2.5 M NaOH(aq) holding at 348 K (75 °C) for 1 to 24 hours, XRD results demonstrated that all samples were HA and no other phases could be detected. Moreover, the XRD results also show that all the as-dried powders still maintained the HA structure when DCPD underwent hydrolysis in 0.1 to 5 M NaOH(aq) at 348 K (75 °C) for 1 hour. Otherwise, the full transformation from HA to octa-calcium phosphate (OCP, Ca8H2(PO4)6·5H2O) occurred when hydrolysis happened in 10 M NaOH(aq). FT-IR spectra analysis revealed that some carbonated HA (Ca10(PO4)6(CO3), CHA) had formed. The SEM morphology results show that the 60 to 65 nm width of the uniformly long rods with regular alignment formed in the HA powder aggregates when DCPD underwent hydrolysis in 2.5 M NaOH(aq) at 348 K (75 °C) for 1 hour.








Similar content being viewed by others

References
G.K. Lim, J. Wang, S.C. Ng, and L.M. Gam. Mater. Lett. 1996, Vol. 28, pp. 431-33.
A.I. Villacampa, and J.M. Garcia-Ruiz. J. Cryst. Growth, 2000, Vol. 211, pp. 111-15.
M.Y. Shareef, P.F. Messer, and R. Van Noort. Biomaterials, 1993, Vol. 14, pp. 69-75.
W. Suchanek, and M. Yoshimura. J. Mater. Res. 1998, Vol. 13, pp. 94-117.
F. Chen, Z.C. Wang, and C.J. Lin. Mater. Lett. 2002, Vol. 57, pp. 858-61.
J. Currey. Nature, 2001, Vol. 414, p. 699.
X. Yang, and Z. Wang. J. Mater. Chem. 1998, Vol. 8, pp. 2233-37.
K. Agrawal, G. Singh, D. Puri, and S. Prakash. J. Miner. Mater. Charater. Eng. 2011, Vol. 10, pp. 727-34.
E.M. Zahrani, M.H. Fathi, and A.M. Alfantazi. Metall. Mater. Trans. A 2011, Vol. 42A, pp. 3291-3309.
S.J. Kalita, and S. Verma. Mater. Sci. Eng. C. 2010, Vol. 30, pp. 295-303.
N.A.M. Barakat, M.S. Khil, A.M. Omran, F.A. Sheikn, and H.Y. Kim. J. Mater. Process. Technol. 2009, Vol. 209, pp. 3408-15.
T.Y. Ma, Z.G. Xia, and L.B. Liao. Appl. Surf. Sci. 2011, Vol. 257, pp. 4384-88.
M. Jarcho, C.H. Bolen, M.B. Thomas, J. Bobik, F. Kay, and R.H. Dorcmus. J. Mater. Sci. 1976, Vol. 11, pp. 2027-35.
K. Ohta, M. Kokuchi, J. Tanaka, and H. Eda. Chem. Lett. 2003, Vol. 9, pp. 894-95.
W.J. Shih, Y.F. Chen, M.C. Wang, and M.H. Hon. J. Cryst. Growth, 2004, Vol. 270, pp. 211-18.
W.J. Shih, M.C. Wang, and M.H. Hon. J. Cryst. Growth, 2005, Vol. 275, pp. e2339-e2344.
G.C. Koumoulidis, A.P. Katsoulidis, A.K. Ladavos, P.J. Pomonis,C.C. Trapalis, A.T. Sdoukos, and T.C. Vaimukis. J. Colloid. Interface Sci. 2003, Vol. 259, pp. 254-60.
S. Ban, and J. Hasegawa. Biomaterials, 2003, Vol. 23, pp. 2965-72.
M.C. Wang, W.J. Shih, K.M. Chang, S.H. Wang, W.L. Li, and H.H. Huang. J. Non-Cryst. Solids, 2010, Vol. 356, pp. 1546-53.
W.J. Shih, M.C. Wang, K.M. Chang, C.L. Wang, S.H. Wang, W.L. Li, and H.H. Huang (2010) Metall. Mater. Trans. A, 41A. pp. 3509-16.
M.T. Fulmer, and P.W. Brown. J. Mater. Sci. Mater. Med. 1998, Vol. 9, pp. 197-202.
E.A.P. Maeyer, R.M.H. Verbeeck, and D.E. Naessns (1993) Inorg. Chem., 32, pp. 5709–14.
H.E. Feki, J.M. Savariault, and A.B. Salah. J. Alloys. Compd. 1999, Vol. 287, pp. 114-20.
C. Liu, Y. Huang, W. Shen, and J. Cui. Biomaterials, 2001, Vol. 22, pp. 301-06.
H.S. Liu, T.S. Chin, S.Y. Chiu, K.H. Chung, C.S. Chang, and M.T. Liu. Ceram. Int. 1997, Vol. 23, pp. 19-25.
M.J. Kohn, J. Rabovan, and J.M. Hughes: in Phosphates: Geochemical, Geobiological, and Materials Importance, P.H. Ribbe, ed., Mineralogical Society of America, Washington, DC, 2002, p. 427.
S. Murakami, K. Kato, Y. Enari, M. Kamitakahara, N. Watanabe, and K. Ioku. Ceram. Int. 2012, Vol. 38, pp. 1649-54.
W.E. Brown. Clin. Orthop. Relat. Res. 1966, Vol. 44, pp. 205-20.
B.B. Tomazic, I. Mayer, and W.E. Brown. J. Cryst. Growth 1991, Vol. 108, pp. 670-82.
M. Mathew, and S. Takagi. Natl Inst. Stand. Technol. 2001, Vol. 106, pp. 1035–44.
K. de Groot (1983) Bioceramics of Calcium Phosphate. CRC Press, Boca Raton, FL, pp. 1-32.
R. Murugan, and S. Ramakrishna. J. Cryst. Growth 2005, Vol. 274, pp. 209-13.
M.C. Chang, and J. Tanaka. Biomaterials, 2002, Vol. 23, pp. 4811-18.
M. Manso, M. Langlet, C. Jiménez, and J.M. Martínez-Duart. Int. J. Inorg. Mater. 2001, Vol. 3, pp. 1153-55.
J.P. Lafon, E. Champion, and D. Bernache. J. Eur. Ceram. Soc. 2008, Vol. 28, pp. 139-47.
D.A. Palmer, and R.V. Eldik. Chem. Rev., 1983, Vol. 83, pp. 651–731.
C.M. Zhang, J. Yang, Z. Quan, P.P. Yang, C. Li, Z.Y. Hou, and J. Lin. Cryst. Growth Des. 2009, Vol. 9, pp. 2725-33.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Science Council of the Republic of China (Taiwan) under contract No. NSC 97-2221-E-037-001-MY3. The authors also gratefully acknowledge the help of Mr. F. C. Wu and Mr. H. Y. Yao with regard to SEM and TEM photography, respectively, when preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted April 9, 2012.
Rights and permissions
About this article
Cite this article
Wang, MC., Hon, MH., Chen, HT. et al. Process Parameters on the Crystallization and Morphology of Hydroxyapatite Powders Prepared by a Hydrolysis Method. Metall Mater Trans A 44, 3344–3352 (2013). https://doi.org/10.1007/s11661-013-1662-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-013-1662-6