Abstract
Summary
Osteosarcopenic obesity (OSO) is a complex disease commonly seen in the elderly. We found that resistance training may improve bone mineral density, skeletal muscle mass, and body fat percentage in patients with OSO. Therefore, resistance training is beneficial for elderly OSO patients and is worth being promoted.
Purpose
Investigate effects of resistance training on body composition and physical function in elderly osteosarcopenic obesity (OSO) patients.
Methods
PubMed, Web of Science, Embase, Cochrane Library, Medline, SinoMed, CNKI, and Wanfang Database were searched from inception until October 13, 2021.Two independent researchers extracted the key information from each eligible study. The methodological quality of included studies was assessed using the Physiotherapy Evidence Database (PEDro) scale. The Cochrane Risk of Bias Tool was used to assess the risk of bias. Grading of Recommendations Assessment Development and Evaluation (GRADE) was used to evaluate the quality of the outcomes. Sensitivity analysis indicated the stability of the results. Statistical analysis was performed using Review Manager 5.3.
Results
Four randomized controlled studies meeting the inclusion criteria were included, with 182 participants. Twelve weeks of resistance training improved bone mineral density (BMD, mean difference (MD) = 0.01 g/cm2, 95% confidence interval (CI): 0.001, 0.02, P = 0.03, I2 = 0%), skeletal muscle mass (SMM, MD = 1.19 kg, 95% CI: 0.50, 1.89, P = 0.0007, I2 = 0%), Z score, timed chair rise test (TCR), and body fat percentage (BFP, MD = − 1.61%, 95% CI: − 2.94, − 0.28, P = 0.02, I2 = 50%) but did not significantly affect skeletal muscle mass index (SMI, MD = 0.20 kg/m2, 95% CI: − 0.25, 0.64, P = 0.38, I2 = 0%) or gait speed (GS).
Conclusions
Resistance training is a safe and effective intervention that can improve many parameters, including BFP, SMM, and Z score, among OSO patients and is a good option for elderly individuals to improve their physical fitness.
Highlights
-
1.
Osteosarcopenic obesity is a combination of osteoporosis, sarcopenia, and obesity.
-
2.
Osteosarcopenic obesity may lead to higher rates of disability and mortality.
-
3.
Resistance training can improve body composition and physical function.
-
4.
Resistance training is beneficial to elderly osteosarcopenic obesity patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid development of the world economy, some countries have become aging societies, and an aging population results in greater health challenges. Aging can lead to some diseases in the elderly, such as sarcopenia [1,2,3,4], obesity [5,6,7], and osteoporosis [8,9,10]. Sarcopenia can cause muscle weakness [11] and increase the risk of falls and fractures [12]. Obesity is an important cause of metabolic syndrome [13] and chronic diseases such as hypertension [14,15,16], hyperlipidemia [17], hyperglycemia [18, 19], and cardiovascular diseases [20,21,22,23]. Osteoporosis is a risk factor for fragility fracture [24, 25]. The concomitant occurrence of all three diseases is referred to as osteosarcopenic obesity (OSO), which places a very large burden on global health [26]. Studies have shown that OSO is associated with a reduced functionality [27, 28], frailty [29] and falls [27] in patients. In 2014, the concept of OSO was first proposed by Ilich et al. that patients suffer from both bone loss, muscle loss, and fat gain [30]. Since then, OSO has gradually attracted people’s attention, and the corresponding diagnostic criteria [31] and treatment principles [32, 33], including nutritional intervention [34,35,36,37] and exercise intervention [38], have been proposed. In 2019, Kelly et al. revised the physical diagnostic criteria for OSO based on the differences in body composition between men and women [39]. However, this diagnostic criterion may only be applicable to European populations and may need to be modified when used to diagnose other ethnic groups.
Exercise, especially resistance training, is considered an effective strategy for the treatment of sarcopenic obesity [40,41,42,43,44] and osteosarcopenia [26, 45]. Sarcopenic obesity is more dangerous than sarcopenia or obesity alone [46] because sarcopenia and obesity may interact with each other to maximize their impact on morbidity, disability, and mortality in older people [46]. And osteosarcopenia is associated with greater health risks than osteoporosis or sarcopenia alone [47]. OSO is a combination of osteoporosis, sarcopenia, and obesity, which may be much more severe than osteoporosis, sarcopenia, or obesity alone [48]. Patients with OSO may have greater health risks than individuals with sarcopenic obesity or osteosarcopenia. As previously mentioned, resistance training is beneficial in improving sarcopenic obesity and osteosarcopenia, so is resistance exercise equally effective for OSO? Currently, only a few small sample clinical studies have been conducted to explore this question but have obtained different results [49,50,51,52,53]. Nevertheless, there has been no systematic review or meta-analysis conducted to evaluate the efficacy of resistance training interventions on OSO.
Consequently, the purpose of this study was to explore the effect of resistance training on parameters related to the physical health and functional performance of elderly patients with OSO, including body composition, physical function, and OSO Z score.
Methods
This article was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [54]. This article has been registered on PROSPERO, the registration number is CRD42021285205.
Search strategies
We searched the published literature on PubMed, Medline, Web of Science, Embase, Cochrane Library, SinoMed, CNKI, and Wanfang Database without imposing publication date or language restrictions. A systematic search was performed for eligible studies published through Oct 13, 2021. The keywords were (osteosarcopeni* OR osteo-sarcopeni* OR sarco-osteopeni* OR sarco-osteoporo*) AND (obesity OR adiposity OR overweight) AND (resistance training OR resistance exercise OR strength training) AND (randomized controlled OR RCT OR controlled trial OR clinical trial). The PubMed search strategy is shown in Table S1. In addition, to prevent the omission of relevant studies, a further manual search of references was performed to identify potential studies that were not captured by database retrieval. For example, we searched the references of included studies to prevent the omission of relevant literature.
Study selection
Two authors (Hua Ye, Jia-hong Zhang) independently selected the literature. If any differences existed, a meeting was held to resolve them. The inclusion criteria for the studies were based on the PICOS (patients, intervention, comparison, outcomes, and study design) principle [55], as shown below:
-
Patients (P): People with OSO (participants were required to have a combination of osteopenia, sarcopenia, and obesity and to have no other diseases, such as fractures, heart failure, and diabetes). Participants were required to be elderly (age ≥ 60), but there were no restrictions for sex or environment (such as hospitals, communities, or nursing homes).
-
Intervention (I): Interventions included resistance training, such as elastic resistance training and progressive resistance training.
-
Comparison (C): Control group or placebo.
-
Outcomes (O): Primary outcomes: body composition (e.g., body fat percentage (BFP), skeletal muscle mass index (SMI), bone mineral density (BMD)); secondary outcomes: physical function (e.g., hand grip strength (HGS), gait speed (GS)) and OSO Z score. Among them, BFP, SMI, and BMD were calculated using data obtained from the dual-energy X-ray absorptiometry (DXA). The HGS of the subject’ s dominant hand was measured using a standard hydraulic hand dynamometer. A 10-m walk test (10 MWT) was measured to obtain the GS of the participants.
-
Study design (S): Only randomized controlled trials were included in this study.
For articles with overlapping data of the same population source, only the largest report was included, unless they reported different outcomes of interest.
Studies were excluded if they met the following criteria: (1) participants were not patients with OSO (for example, patients with just osteoporosis, sarcopenia or obesity, or with other diseases, such as fractures or heart failure); (2) interventions did not include resistance training; (3) the comparisons were not performed according to the intervention type; (4) outcomes did not include body composition, physical function, or OSO Z score; and (5) the type of studies were not randomized controlled trials.
Data extraction
Two authors (Qin-qin Liu, Qiang Zhu) independently extracted the data from the selected studies into a Microsoft Excel spreadsheet and then summarized the data in a table. Any disagreement was resolved in a consensus meeting.
The data extracted included (1) first author and year of publication; (2) country or region of author; (3) sex and age of participants; (4) groups and sample size; (5) time points of outcome assessment; (6) total intervention time; (7) outcome indicators; and (8) Physiotherapy Evidence Database (PEDro) scores for each study (as shown in Table 1). In addition, we collected information on the measurement tools used to assess body composition and diagnostic criteria of OSO for each study (as shown in Table 2).
Quality assessment
Two authors (Hui Huang, Hui-yong Xie) independently completed an assessment of the methodological quality of each included study using the PEDro scale. If the score was inconsistent between the two authors, a third author (Yi Long) was consulted to judge the final score. The PEDro scale has 11 items: (1) eligibility criteria and source, (2) random allocation, (3) concealed allocation, (4) baseline comparability, (5) participant blinding, (6) therapist blinding, (7) assessor blinding, (8) adequate follow-up (> 85%), (9) intention-to-treat analysis, (10) between-group statistical comparisons, and (11) point and variability measurements. A total PEDro score is achieved by adding the ratings of items (2) to (11) for a combined total score between 0 and 10. Scores of less than 4 are considered poor, 4 to 5 are considered fair, 6 to 8 are considered good, and 9 to 10 are considered excellent [56].
In addition, we assessed the quality of each outcome according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) criteria [57] by assigning ratings such as “very low,” “low,” “moderate,” and “high,” which involved evaluating eight domains, including risk of bias, directness of evidence, consistency and precision of results, publication bias, magnitude of effect, dose–response, and influence of confounding factors.
Risk of bias
Two authors (Hui Huang, Hui-yong Xie) assessed the risk of bias of the included studies using the Cochrane Risk of Bias Tool [58]. If there was a disagreement, a third author (Yi Long) was involved to reach a consensus. There are seven items in the bias risk table: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); and (7) other bias. Each item was classified as low risk, high risk (not fulfilling the criteria), or unclear risk (specific details or descriptions were not reported). Furthermore, the presence of publication bias was estimated using a funnel plot.
Data synthesis and statistical analyses
Statistical analysis was performed using Review Manager 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). The pre-extracted mean value, standard deviation, and sample size were input into the statistical software. In our meta-analyses, we reported the effect size using the mean difference (MD) with 95% confidence interval (95% CI) for studies that used the same measurement methods and the standardized mean difference (SMD) for those that measured the same outcome with different units for continuous outcomes. In all analyses, I2 statistics were used to analyze heterogeneity between studies. If the P value of the heterogeneity test (I2 statistic) was < 0.05, the random-effects model was used; otherwise, a fixed-effects model was used. For small sample studies and highly heterogeneous outcomes, sensitivity analysis was used to test the stability of the results.
Results
Study selection
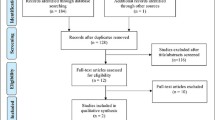
After a systematic search of eight databases, we identified a total of 49 articles, including 7 in PubMed, 19 in Medline, 2 in Embase, 3 in Web of Science, 8 in Cochrane Library, 5 in CNKI, 4 in Wanfang Database, and 1 in SinoMed. No literature was obtained from other sources. After deleting duplicate studies, 25 studies remained. After reading the titles and abstracts, 18 articles were excluded because they were not studies on OSO, 1 study was not an RCT, and the other one was not available, leaving 5 articles. Then, we read the full text of the 5 articles. We noticed that there were two studies that had the same population source and with repeated data on body composition [49, 53]. In addition, Banitalebi et al. (2020) [53] also included physical function indicators, so we included the study with more complete data [53]. The list of literatures exclusion and reasons for exclusion are shown in Table S2. As a result, four studies were included for qualitative analysis [50,51,52,53]. Hence, we finally included only four studies in the meta-analysis [50,51,52,53] (Fig. 1).
Study characteristics
Among the four included studies, three studies involved only older women [50, 51, 53], and the other study included both elderly men and women [52]. One study divided resistance training into two groups based on exercise intensity: G1S (participants only performed 1 set of 10–15 repetitions maximum for each exercise) and G3S (participants performed 3 sets of 10–15 repetitions maximum for each exercise) [50]. In three studies, resistance training was conducted by using elastic bands [51,52,53]. In the remaining study, aerobic exercise was combined with resistance training in the intervention group of patients with OSO [52]. In one study, parameter evaluation was performed in weeks 1–2 and 15–16 for baseline and postintervention [50], and the assessments in the other studies were performed at baseline and postintervention [51,52,53]. The total intervention duration in all four studies was 12 weeks, and resistance training was performed three times every week.
Regarding the assessment tool of body composition, two studies used only DXA to assess body composition [50, 53], and two studies used both DXA and bioelectrical impedance analysis (BIA) [51, 52]. The diagnostic criteria for OSO were divided into three components: osteopenia, sarcopenia, and obesity. Among them, the diagnostic criteria for osteopenia, sarcopenia, and obesity in one study were as follows: (1) osteopenia: − 2.5 ≤ T-score ≤ − 1.0 of L1–L4 and/or total femur or femoral neck; (2) sarcopenia: 10 MWT ≤ 1 (m/s2), and SMI ≤ 28% or ≤ 7.76 kg/m2; and (3) obesity: BFP > 32%, body mass index (BMI) > 30 kg/m2 [53]. One study used a T-score < − 1.0 of L1–L4 to diagnose osteopenia; SMI < 5.67 kg/m2 and a grip strength of < 20 kg or gait speed of < 0.8 m/s to diagnose sarcopenia; and BFP > 35% to diagnose obesity [51]. In the other study, the inclusion criteria were (1) osteopenia: T-score < − 1.0; (2) sarcopenia: men: SMI ≤ 7.0 kg/m2, women: SMI ≤ 5.4 kg/m2; and (3) obesity: men: BFP > 25%, women: BFP > 35% [52]. One study did not mention the diagnostic criteria for OSO in the original text [50] (Table 2).
All the authors of the included studies declared that there was no conflict of interest. One study was funded by the Shahrekord University (grant no. 96INT8M895) [53], and the other was supported by the National Science Council of Taiwan (grant no. NSC 102–2314-B-038–053-MY3) and Taipei Medical University-Wan Fang Hospital, Taiwan (grant no. 98TMU-WFH-05–3) [51].
Resistance training protocol
Three studies used elastic bands for resistance training [51,52,53], and one study combined aerobic exercise with resistance training [52]. The resistance training sessions lasted 60 min in one study [53], 40 min in one study [51], and 45 ~ 60 min in the other study [52]. In addition, in another study, the timing of the intervention was related to grouping: the duration of training for the G1S group was 30 min, while that for the G3S group was 50 min [50]. In all four studies, resistance exercise interventions were performed three times per week (such as on Mondays, Wednesdays, and Fridays) for 12 weeks (Table S3).
Quality of included studies
The PEDro scores for each included study are shown in Table 1, and details of the scores are shown in Table S4. The four studies scored between 5 and 8, with a mean and standard deviation of 6.75 ± 1.258. Only one of the studies scored 5, and the quality of the literature was considered fair [52], while the remaining three scored 7 or 8 and were considered good [50, 51, 53]. All of the included studies had baseline comparability, between-group statistical comparisons, and point and variability measurements. In addition, concealed allocation was used in three studies [50, 51, 53], assessor blinding was used in three studies [50, 51, 53], follow-up rates were greater than 85% in three studies [50,51,52], and intention-to-treat analysis was used in two studies [51, 53]. One study did not mention the use of blinding in the original text [52].
Risk of bias of included studies
The risk of bias assessment for four studies is shown in Fig. 2 according to the Cochrane tool. One study did not mention allocation concealment (unclear risk of bias) [52]. Neither participants nor researchers were blinded due to the nature of the intervention in two studies (low risk of bias) [51, 53], while two studies did not mention participants or researchers blinding (unclear risk of bias) [50, 52]. One study had incomplete outcome data because the follow-up rates were lower than 85% (high risk of bias) [53]. One study used not only resistance training but also aerobic exercise as an intervention [52], which may have an impact on the final results and result in a risk of bias (high risk of bias). No studies had selection bias or reporting bias. Due to the insufficient number of included studies, a funnel plot analysis was not performed.
Effects of resistance training on primary outcomes
Four studies (n = 182) investigated the effects of resistance exercise on BMD and BFP [50,51,52,53]. Two studies (n = 79) assessed skeletal muscle mass (SMM) [50, 51], and two studies (n = 57) measured SMI [51, 52].
Forest plot results showed that resistance training can effectively increase BMD (MD = 0.01 g/cm2, 95% CI: 0.001, 0.02, P = 0.03, I2 = 0%), significantly increase SMM (MD = 1.19 kg, 95% CI: 0.50, 1.89, P = 0.0007, I2 = 0%), and decrease BFP (MD = − 1.61%, 95% CI: − 2.94, − 0.28, P = 0.02, I2 = 50%) but that it has no effect on SMI (MD = 0.20 kg/m2, 95% CI: − 0.25, 0.64, P = 0.38, I2 = 0%) (Fig. 3).
Effects of resistance training on secondary outcomes
Because only one study could extract available data for secondary outcomes, it was not possible to conduct a meta-analysis. As shown in Table 1, two studies explored the OSO Z score, and the results showed that resistance training can effectively improve the Z score [50, 53]. They treated the OSO Z score as a composite Z score derived from the average of the components calculated based on the following formula: Z score = ((muscular strength Z score) + (SMM Z score) + (− 1 × body fat Z score) + (BMD Z score))/4 [50, 53]. Two studies measured HGS, GS, timed up and go (TUG), and 30-s timed chair rise (TCR) repetition and showed that 12 weeks of elastic band resistance training significantly improved TCR but had no effect on GS [51, 53]. However, the two studies showed opposite results for HGS and TUG. Banitalebi et al.[53] found that 12 weeks of resistance training increased HGS (P = 0.013) but had no effect on TUG (P = 0.225). Nevertheless, Lee et al. [51] showed the opposite result: progressive elastic band resistance exercise training could not improve HGS (P = 0.413) but could increase TUG (P = 0.030).
Quality of outcome indicators
The quality of the evidence as assessed using GRADE was rated from very low to high. All outcomes were imprecise because the sample sizes included in the studies were too small. The BMD results may have a serious risk of bias because the intervention in the study by Li et al. [52] was resistance training combined with aerobic exercise, and these results were heavily weighted in the BMD analysis. BFP, HGS, and TUG may have serious inconsistencies because of opposite results or high heterogeneity across studies. As a result, for primary outcomes, the quality of evidence for SMI and SMM was moderate, while that of BMD and BFP was low. Regarding secondary outcomes, the quality of evidence for GS, TCR, and OSO Z score was moderate, but that for HGS and TUG was low (Table 3).
Sensitivity analysis
To test the stability of the meta-analysis results, we performed a sensitivity analysis on the primary outcomes. As seen in the forest plot, the study of Li et al. [52] accounted for 96.7% of the weight in BMD, and we found that the BMD result became meaningless when the study was removed (MD = 0.02 g/cm2, 95% CI: − 0.02, 0.06, P = 0.37, I2 = 0%). For BFP, when the study of Banitalebi et al. [53] was removed, the heterogeneity became 0%, and the results became more significant (MD = − 4.31%, 95% CI: − 6.61, − 2.01, P = 0.0002, I2 = 0%).
Discussion
In this systematic review and meta-analysis, we included only randomized controlled studies. The purpose of this study was to investigate the effects of resistance training on body composition and physical function in elderly OSO patients. We found that 12 weeks of elastic band resistance training can effectively increase BMD, significantly improve SMM, and distinctly reduce BFP in older people with OSO but had no effect on SMI. In addition, resistance training effectively improved OSO Z score and TCR but not GS. Regarding the influence of resistance training on HGS and TUG, two studies had different results. And as there are not enough data for a meta-analysis of these two outcomes, no definitive conclusions can yet be drawn.
Although our study showed that resistance training can increase BMD, of the 4 studies included in the meta-analysis for BMD, only Li et al. demonstrated a significant increase in BMD [52]; the other three studies showed no significant difference between the experimental and control groups. In addition, it is worth noting that the intervention method in the study by Li et al. [52] was resistance training combined with aerobic exercise, so it is impossible to determine how much benefit of its influence on BMD was derived from resistance training; therefore, we should be cautious when interpreting this result. We noted that the merged data result of BFP had moderate heterogeneity (I2 = 50%). Through sensitivity analysis, we found that the source of heterogeneity was the study conducted by Banitalebi et al. [53], and the heterogeneity may be a result of their definition of obesity and the characteristics of the participants. Overall, the four randomized controlled studies included in this study had a low risk of bias, the GRADE evidence for outcomes was rated as low (four outcomes) and moderate (five outcomes), and only one outcome was moderately heterogeneous and acceptable. Therefore, our conclusions may provide some reference for the development of clinical guidelines.
Osteoporosis, sarcopenia, and obesity were once considered separate diseases and were rarely studied together. Later, people combined two of these conditions, sarcopenia and obesity [59, 60], as well as osteoporosis and sarcopenia [61, 62], and explored them. OSO is a syndrome of osteoporosis, sarcopenia, and obesity that was first proposed in 2014 and indicates a link between bone, muscle, and fat [30]. The diagnosis of OSO should be carried out from two aspects: physical via body composition measurements and functional via physical performance measures [31]. Body composition can be measured in three ways: BMD T-score to check for osteoporosis, appendicular lean mass to identify sarcopenia, and BFP to determine obesity [31]. At present, the assessment tools for body composition mainly include DXA and BIA. Physical performance tests, such as HGS, single leg stance, and GS, could be assessed with minimal equipment [51].
The safety of the intervention is a key issue that researchers must consider. With the exception of one study that reported that a small number of patients in the experimental group reported muscle soreness, knee pain, and shoulder pain (25%) during the first three sessions of training [53], no other studies reported adverse events, suggesting that resistance training is a safe intervention for older adults.
To our knowledge, this is the first systematic review and meta-analysis of the effects of resistance training on elderly patients with OSO. The results of this study indicate that resistance training has a significant beneficial effect in the treatment of OSO in many aspects, and this effect is affected by the intensity of resistance training; the higher the intensity is, the greater the effect. Nevertheless, in one of the included studies, the intervention method combined aerobic exercise with resistance training [52], so we cannot be sure how much of the final intervention benefit was due to resistance training, which may be a source of bias.
In addition, this study had some limitations. First, the number of studies included in this analysis was very limited, with only four small samples, which may have some impact on the accuracy of the outcomes. Second, the intervention time in all four studies was short, at just 12 weeks. Therefore, future studies with large samples and long intervention durations are needed to verify these results.
In summary, resistance training is a safe and effective intervention for elderly patients with OSO. The government should vigorously promote resistance training, and the public should also actively respond, which may have a good effect on the prevention of OSO. Resistance training may also reduce some of the economic burden resulting from OSO.
Conclusions
Resistance training is a safe and effective intervention that can improve many parameters in OSO patients, such as BFP and SMM. Resistance training is a good option for elderly individuals who want to improve their physical fitness.
Data availability
Published data used for the systematic review and meta-analysis are available from the authors.
References
Morley JE (2016) Frailty and sarcopenia in elderly. Wien Klin Wochenschr 128:439–445
Fuggle N, Shaw S, Dennison E, Cooper C (2017) Sarcopenia. Best Pract Res Clin Rheumatol 31:218–242
Cruz-Jentoft AJ, Sayer AA (2019) Sarcopenia. Lancet 393:2636–2646
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Kalish VB (2016) Obesity in older adults. Prim Care 43:137–144, ix
Mathus-Vliegen EM (2012) Obesity and the elderly. J Clin Gastroenterol 46:533–544
Cetin DC, Nasr G (2014) Obesity in the elderly: more complicated than you think. Cleve Clin J Med 81:51–61
Lane JM, Russell L, Khan SN (2000) Osteoporosis. Clin Orthop Relat Res (372):139–150. https://doi.org/10.1097/00003086-200003000-00016
Black DM, Rosen CJ (2016) Clinical practice. Postmenopausal Osteoporosis. N Engl J Med 374:254–262
Ensrud KE, Crandall CJ (2017) Osteoporosis. Ann Intern Med 167:Itc17-32
Tieland M, Trouwborst I, Clark BC (2018) Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 9:3–19
Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB (2019) Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 10:485–500
Després JP, Lemieux I (2006) Abdominal obesity and metabolic syndrome. Nature 444:881–887
Chen X, Kong C, Yu H, Gong J, Lan L, Zhou L, Gong J, Liu P, Xu L, Deng Q (2019) Association between osteosarcopenic obesity and hypertension among four minority populations in China: a cross-sectional study. BMJ Open 9:e026818
Seravalle G, Grassi G (2017) Obesity and hypertension. Pharmacol Res 122:1–7
Mendoza MF, Kachur SM, Lavie CJ (2020) Hypertension in obesity. Curr Opin Cardiol 35:389–396
Mo D, Hsieh P, Yu H, Zhou L, Gong J, Xu L, Liu P, Chen G, Chen Z, Deng Q (2018) Osteosarcopenic obesity and its relationship with dyslipidemia in women from different ethnic groups of China. Arch Osteoporos 13:65
Wakabayashi I, Daimon T (2012) Age-dependent decline of association between obesity and hyperglycemia in men and women. Diabetes Care 35:175–177
Lu Y, Wang E, Chen Y et al (2020) Obesity-induced excess of 17-hydroxyprogesterone promotes hyperglycemia through activation of glucocorticoid receptor. J Clin Investig 130:3791–3804
Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO (2017) Obesity and cardiovascular diseases. Minerva Med 108:212–228
Ortega FB, Lavie CJ, Blair SN (2016) Obesity and cardiovascular disease. Circ Res 118:1752–1770
Csige I, Ujvárosy D, Szabó Z (2018) The impact of obesity on the cardiovascular system. J Diabetes Res 2018:3407306
Ortega-Loubon C, Fernández-Molina M, Singh G, Correa R (2019) Obesity and its cardiovascular effects. Diabetes Metab Res Rev 35:e3135
Coughlan T, Dockery F (2014) Osteoporosis and fracture risk in older people. Clin Med (Lond) 14:187–191
Johnston CB, Dagar M (2020) Osteoporosis in older adults. Med Clin North Am 104:873–884
Kirk B, Zanker J (2020) Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle 11:609–618
Hita-Contreras F, Martínez-Amat A, Cruz-Díaz D, Pérez-López FR (2015) Osteosarcopenic obesity and fall prevention strategies. Maturitas 80:126–132
Ilich JZ, Inglis JE, Kelly OJ, McGee DL (2015) Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos Int 26:2587–2595
Szlejf C, Parra-Rodríguez L, Rosas-Carrasco O (2017) Osteosarcopenic obesity: prevalence and relation with frailty and physical performance in middle-aged and older women. J Am Med Dir Assoc 18:733.e731-733.e735
Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ (2014) Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev 15:51–60
Ilich JZ, Kelly OJ (2016) Osteosarcopenic obesity syndrome: what is it and how can it be identified and diagnosed? Curr Gerontol Geriatr Res 2016:7325973
Kelly OJ, Gilman JC, Boschiero D, Ilich JZ (2019) Osteosarcopenic obesity: current knowledge, revised identification criteria and treatment principles. Nutrients 11:747
García-González AI, Axtle-Serrano Z, López-Teros M, Szlejf C, Martínez-Ruiz A, Rosas-Carrasco O (2018) Clinical interventions in osteosarcopenic obesity: nutrition, physical and psychological activity. Rev Med Inst Mex Seguro Soc 56:S82-93
Bae YJ (2020) Fruit intake and osteosarcopenic obesity in Korean postmenopausal women aged 50–64 years. Maturitas 134:41–46
Kelly OJ, Gilman JC, Kim Y, Ilich JZ (2016) Micronutrient intake in the etiology, prevention and treatment of osteosarcopenic obesity. Curr Aging Sci 9:260–278
Kelly OJ, Gilman JC, Kim Y, Ilich JZ (2017) Macronutrient intake and distribution in the etiology, prevention and treatment of osteosarcopenic obesity. Curr Aging Sci 10:83–105
Choi MK, Bae YJ (2020) Protein intake and osteosarcopenic adiposity in Korean adults aged 50 years and older. Osteoporos Int 31:2363–2372
Kelly OJ, Gilman JC (2017) Can unconventional exercise be helpful in the treatment, management and prevention of osteosarcopenic obesity? Curr Aging Sci 10:106–121
Kelly OJ, Gilman JC, Boschiero D, Ilich JZ (2019) Osteosarcopenic Obesity: Current knowledge, revised identification criteria and treatment principles. Nutrients 11(4):747. https://doi.org/10.3390/nu11040747
Hsu KJ, Liao CD, Tsai MW, Chen CN (2019) Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: a meta-analysis. Nutrients 11:2163
Batsis JA, Villareal DT (2018) Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 14:513–537
Petroni ML, Caletti MT, Dalle Grave R (2019) Prevention and treatment of sarcopenic obesity in women. Nutrients 11:1302
Martínez-Amat A, Aibar-Almazán A, Fábrega-Cuadros R, Cruz-Díaz D, Jiménez-García JD, Pérez-López FR, Achalandabaso A, Barranco-Zafra R, Hita-Contreras F (2018) Exercise alone or combined with dietary supplements for sarcopenic obesity in community-dwelling older people: a systematic review of randomized controlled trials. Maturitas 110:92–103
Theodorakopoulos C, Jones J, Bannerman E, Greig CA (2017) Effectiveness of nutritional and exercise interventions to improve body composition and muscle strength or function in sarcopenic obese older adults: a systematic review. Nutr Res 43:3–15
Atlihan R, Kirk B, Duque G (2021) Non-pharmacological interventions in osteosarcopenia: a systematic review. J Nutr Health Aging 25:25–32
Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V (2008) Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 18:388–395
Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R (2016) Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res 28:895–899
Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A, Panton L (2014) Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle 5:183–192
Banitalebi E, Ghahfarrokhi MM, Dehghan M (2021) Effect of 12-weeks elastic band resistance training on MyomiRs and osteoporosis markers in elderly women with osteosarcopenic obesity: a randomized controlled trial. BMC Geriatr 21:433
Cunha PM, Ribeiro AS, Tomeleri CM, Schoenfeld BJ, Silva AM, Souza MF, Nascimento MA, Sardinha LB, Cyrino ES (2018) The effects of resistance training volume on osteosarcopenic obesity in older women. J Sports Sci 36:1564–1571
Lee YH, Lee PH, Lin LF, Liao CD, Liou TH, Huang SW (2021) Effects of progressive elastic band resistance exercise for aged osteosarcopenic adiposity women. Exp Gerontol 147:111272
李申, 黄力平, 王磊, 陈英武, 李林珂, 张琳, 曹龙军, 刘畅格, 王静, 袁士磊 (2020) 12周有氧步行联合快速力量训练对肌骨减少性肥胖老年人干预效果研究. 中国康复医学杂志 35:420-426 Shen LI, Huang L, Wang L, Chen Y, Li L, Zhang L, Cao L, Liu C, Wang J, Yuan S (2020) Effects of 12 weeks aerobic exercise combined with high speed strength training on old adults with osteosarcopenic obesity syndrome. Chin J Rehabil Med 35:420-426
Banitalebi E, Faramarzi M, Ghahfarokhi MM, SavariNikoo F, Soltani N, Bahramzadeh A (2020) Osteosarcopenic obesity markers following elastic band resistance training: a randomized controlled trial. Exp Gerontol 135:110884
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16
Cashin AG, McAuley JH (2020) Clinimetrics: physiotherapy evidence database (PEDro) scale. J Physiother 66:59
Atkins D, Best D, Briss PA et al (2004) Grading quality of evidence and strength of recommendations. BMJ 328:1490
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Kalinkovich A, Livshits G (2017) Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 35:200–221
Zamboni M, Rubele S, Rossi AP (2019) Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care 22:13–19
Clynes MA, Gregson CL, Bruyère O, Cooper C, Dennison EM (2021) Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology (Oxford) 60:529–537
Inoue T, Maeda K, Satake S, Matsui Y, Arai H (2022) Osteosarcopenia, the co-existence of osteoporosis and sarcopenia, is associated with social frailty in older adults. Aging Clin Exp Res 34(3):535–543. https://doi.org/10.1007/s40520-021-01968-y
Funding
Mao-yuan Wang is supported by the National Natural Science Foundation of China (grant numbers 82060420). The funders have no role in study design, data collection or analysis, preparation of the manuscript, or the decision to publish. The contents of this publication are solely the responsibility of authors and do not necessarily represent the official views of the funding sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Jm., Ye, H., Zhu, Q. et al. Effects of resistance training on body composition and physical function in elderly patients with osteosarcopenic obesity: a systematic review and meta-analysis. Arch Osteoporos 17, 82 (2022). https://doi.org/10.1007/s11657-022-01120-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-022-01120-x