Abstract
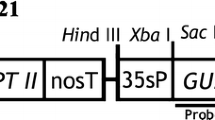
Limonium bicolor, a typical recretohalophyte, has a specialized salt-secreting structure in the epidermis called the salt gland and plays a significant role in improving saline land. Understanding the molecular mechanisms of salt secretion and salt gland development requires an efficient L. bicolor transformation system, which is described in this report. Leaf explants were incubated with Agrobacterium tumefaciens strain EHA105 harboring the plasmid pTCK303 containing the β-glucuronidase gene (GUS) as the transgene reporter and the hygromycin B resistance gene as a selectable marker. Up to 96.9% of leaves were induced to regenerate shoots on an Murashige and Skoog (MS) medium supplemented with 4.4 μM 6-benzyladenine and 1.1 μM α-naphthaleneacetic acid; roots were induced on the MS medium containing 2.5 μM indole-3-butyric acid. This tissue culture system was suitable for Agrobacterium-mediated transformation of L. bicolor. Pre-cultivated explants (2 d old) were incubated with Agrobacterium (0.6–0.7 at OD600) in a shaking culture for 20 min; the explants and bacterium were co-cultivated for 4 d in the dark before the explants were transferred to a selection medium containing 8 mg/L hygromycin B and 600 mg/L piperacillin sodium (added to prevent continued Agrobacterium growth). Histochemical assays and PCR to detect the GUS gene showed that transformation frequency was 4.43%. Quantitative PCR and Northern blotting further verified the integration and presence of the GUS gene in L. bicolor. This is the first report of an Agrobacterium-based transformation system for L. bicolor. The system will facilitate a research on the identity and function of genes involved in salt gland development and salt secretion.






Similar content being viewed by others
References
Ban QY, Liu GF, Wang YC (2011) A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco. J Plant Physiol 168:449–458
Chai ML, Jia YF, Chen S, Gao ZS, Wang HF, Liu LL, Wang PJ, Hou DQ (2011) Callus induction, plant regeneration, and long-term maintenance of embryogenic cultures in Zoysia matrella [L.] Merr. Plant Cell Tiss Organ Cult 104:187–192
Chen J, Xiao Q, Wu FH, Dong XJ, He JX, Pei ZM, Zheng HL (2010) Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H+-ATPase and Na+/H+ antiporter under high salinity. Tree Physiol 30:1570–1585
Dai H, Zhang Z, Guo X (2007) Adventitious bud regeneration from leaf and cotyledon explants of Chinese hawthorn (Crataegus pinnatifida Bge. var. major N.E.Br.). In Vitro Cell Dev Biol Plant 43:2–8
Diao GP, Wang YC, Yang CP (2010) Functional characterization of a gluthathione S-transferase gene from Limonium bicolor in response to several abiotic stresses. Afr J Biotechnol 9:5060–5065
Ding F, Chen M, Sui N, Wang BS (2009) Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. S Afr J Bot 76:95–101
Ding F, Yang JC, Yuan F, Wang BS (2010) Progress in mechanism of salt excretion in recretohalophytes. Front Biol 5:164–170
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Foolad MR, Arulsekar S, Rodriguez RL (1995) Applications of polymerase chain reaction (PCR) in plant genome analysis. In: Gamborg OL, Phillips GC (eds) Plant cell, tissue and organ culture (fundamental methods). Springer, Berlin, pp 281–298
Fukuoka H, Ogawa T, Mitsuhara I, Iwai T, Isuzugawa K, Nishizawa Y, Gotoh Y, Nishizawa Y, Tagiri A, Ugaki M, Ohshima M, Yano H, Murai N, Niwa Y, Hibi H, Ohashi Y (2000) Agrobacterium-mediated transformation of monocot and dicot plants using the NCR promoter derived from soybean chlorotic mottle virus. Plant Cell Rep 19:815–820
Ge XJ, Chu ZH, Lin YJ, Wang SP (2006) A tissue culture system for different germplasms of indica rice. Plant Cell Rep 25:392–402
Hamada A, Shono M, Xia T, Ohta M, Yasuyuki H, Tanaka A, Hayakawa T (2001) Isolation and characterization of a Na+/H+ antiporter gene from the halophyte Atriplex gmelini. Plant Mol Biol 46:35–42
Hansen G, Wright MS (1999) Recent advances in the transformation of plants. Trends Plant Sci 4:226–231
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Ke J, Khan R, Johnson T, Somers DA, Das A (2001) High efficiency gene transfer to recalcitrant plants by Agrobacterium tumefaciens. Plant Cell Rep 20:150–156
Kubota A, Ishizaki K, Hosaka M, Kohchi T (2013) Efficient Agrobacterium-mediated transformation of the liverwort Marchantia polymorpha using regenerating Thalli. Biosci Biotechnol Biochem 77:167–172
Lee K, Jeon H, Kim M (2002) Optimization of a mature embryo-based in vitro culture system for high-frequency somatic embryogenic callus induction and plant regeneration from japonica rice cultivars. Plant Cell Tiss Organ Cult 71:237–244
Li JF, Park E, Arnim AG, Nebenführ A (2009) The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 5:6
Li Y (2008) Kinetics of the antioxidant response to salinity in the halophyte Limonium bicolor. Plant Soil Environ 54:493–497
Liu ZH, Yang CP, Qi XT, Xiu LL, Wang YC (2010) Cloning, heterologous expression, and functional characterization of a chitinase gene, Lbchi32, from Limonium bicolor. Biochem Genet 48:669–679
Munns R, Tester M (2008) Mechanisms of saline tolerance. Annu Rev Plant Biol 59:651–681
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:47–497
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasin N, Estelle M, Voinnet O, Jones JDG (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 12:436–439
Ogawa Y, Mii M (2005) Evaluation of 12 β-lactam antibiotics for Agrobacterium-mediated transformation through in planta antibacterial activities and phytotoxicities. Plant Cell Rep 23:736–743
Schönenberger C, Schütz A, Franco-Obregón A, Zenobi-Wong M (2011) Efficient electroporation of peptides into adherent cells: investigation of the role of mechano-growth factor in chondrocyte culture. Biotechnol Lett 33:883–888
Stafford CA, Walker GP, Ullman DE (2011) Infection with a plant virus modifies vector feeding behavior. Proc Natl Acad Sci U S A 108:9350–9355
Tingay S, McElroy D, Kalla R, Fieg S, Wang M, Thornton S, Brettell R (1997) Agrobacterium tumefaciens-mediated barley transformation. Plant J 11:1369–1376
Tripathi L, Tripathi JN, Hughes YJ’A (2005) Agrobacterium-mediated transformation of plantain (Musa spp.) cultivar Agbagba. Afr J Biotechnol 4:1378–1383
Vergauwe A, Van-Geldre E, Inze D, Van Montagu M, Van-den Eeckhout E (1998) Factors influencing Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. Plant Cell Rep 18:105–110
Wang MB, Waterhouse PM (1997) A rapid and simple method of assaying plants transformed with hygromycin or PPT resistance genes. Plant Mol Biol Report 15:209–215
Wang Z, Chen C, Xu Y, Jiang R, Han Y, Xu Z, Chong K (2004) A practical vector for efficient knockdown of gene expression in rice (Oryza sativa L.). Plant Mol Biol Report 22:409–417
Yang L, Ding J, Zhang C, Jia JW, Weng HB, Liu WX, Zhang DB (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23:759–763
Zhao SZ, Ruan Y, Sun HZ, Wang BS (2008) Highly efficient Agrobacterium-based transformation system for callus cells of the C3 halophyte Suaeda salsa. Acta Physiol Plant 30:729–736
Zhao SZ, Sun HZ, Gao Y, Sui N, Wang BS (2011) Growth regulator-induced betacyanin accumulation and dopa-4,5-dioxygenase (DODA) gene expression in euhalophyte Suaeda salsa calli. In Vitro Cell Dev Biol Plant 47:391–398
Acknowledgments
This work has been supported by the NSFC (National Natural Science Research Foundation of China, project no. 30870138 and no. 31070158), key projects in the National Science & Technology Pillar Program during the 11th 5-yr plan period (2009BADA7B05) and by the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province. We thank Prof. Kang Chong (Institute of Botany, Chinese Academy of Sciences) for kindly providing the plasmid pTCK303.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: John W. Forster
Rights and permissions
About this article
Cite this article
Yuan, F., Chen, M., Yang, J. et al. A system for the transformation and regeneration of the recretohalophyte Limonium bicolor . In Vitro Cell.Dev.Biol.-Plant 50, 610–617 (2014). https://doi.org/10.1007/s11627-014-9611-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-014-9611-7