Abstract
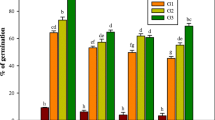
A regeneration system was developed for oriental lily (Lilium orientalis) based on both leaf and bulb scale. Adventitious shoots were regenerated from leaves of in vitro cultures on Murashige and Skoog medium containing thidiazuron (TDZ) or 6-benzylaminopurine (BA) and naphthaleneacetic acid (NAA). The highest percent regeneration from leaf explants was 74.2%, being observed on medium containing 10.8 μM TDZ and 0.54 μM NAA. The highest mean number of shoots generated was 4.4 and was obtained from bulb scale explants on medium containing 0.54 μM TDZ and 0.54 μM NAA. Adventitious shoots were successfully rooted at rates ranging from 79.2% to 100%. The rooted plantlets survived after acclimatization in the greenhouse. The effect of kanamycin concentration on adventitious shoot regeneration was also evaluated, a value of 100 mg l−1 being suggested as a lethal dose for lily transformation. Eighteen ISSR markers were employed to determine the genetic stability of the regenerated shoots in comparison to their mother plant. Eleven primers in total produced 70 clear and reproducible bands. Genetic similarity indicators among the clonal derivatives and the mother plant ranged from 0.92 to 1.0. All 15 micropropagated progenies and the mother plant could be grouped together in one major cluster with a similarity level of 92%. The somaclonal variation rate across the plantlets was estimated as 4.2%, indicating that direct shoot formation from explant regeneration is a safe method for multiplication of “true-to-type” plants.



Similar content being viewed by others
References
Al-Zahim MA, Ford-Lloyd BV, Newbury HJ (1999) Detection of somaclonal variation in garlic (Allium sativum L.) using RAPD and cytological analysis. Plant Cell Rep 18:473–477
Amin MN, Jaiswal VS (1987) Rapid clonal propagation of guava through in vitro shoot proliferation on nodal explants of mature trees. Plant Cell Tissue Organ Cult 9:235–243
Bacchetta L, Remotti PC, Bernardini C, Saccardo F (2003) Adventitious shoot regeneration from leaf explants and stem nodes of Lilium. Plant Cell Tissue Organ Cult 74:37–44
Bhatia R, Singh KP, Jhang T, Sharma TR (2009) Assessment of clonal fidelity of micropropagated gerbera plants by ISSR markers. Sci Hortic 119:208–211
Bhattacharya S, Bandopadhyay TK, Ghosh PD (2010) Somatic embryogenesis in Cymbopogon pendulus and evaluation of clonal fidelity of regenerants using ISSR marker. Sci Hortic 123:505–513
Blair MW, Panaud O, McCouch SR (1999) Inter-simple sequence repeat (ISSR) amplification for analysis of microsatellite motif frequency and fingerprinting in rice (Oryza sativa L.). Theor Appl Genet 98:780–792
Goto S, Thakur RC, Ishii K (1998) Determination of genetic stability in long-term micropropagated shoots of Pinus thunbergii Parl. using RAPD markers. Plant Cell Rep 18:193–197
Hirochika H (1993) Activation of tobacco retrotransposons during tissue culture. EMBO J 12:2521–2528
Irifune K, Morimoto Y, Uchihama M (2003) Production of herbicide-resistant transgenic lily plants by particle bombardment. J Japanese Soc Hort Sci 72:511–516
Joshi P, Dhavan V (2007) Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. Biol Plant 51:22–26
Kumar S, Kashyap M, Sharma DR (2005) In vitro regeneration and bulblet growth from lily bulbscale explants as affected by retardants, sucrose and irradiance. Biol Plant 49:629–632
Lakshman V, Venkataramareddy SR, Neelwarne B (2007) Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron J Biotechnol 10:1–8
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Leroy XJ, Leon K, Charles G, Branchard M (2000) Cauliflower somatic embryogenesis and analysis of regenerant stability by ISSR. Plant Cell Rep 19:1102–1107
Lu Y, Zhang X, Pu J, Qi Y, Xie Y (2011) Molecular assessment of genetic identity and genetic stability in banana cultivars (Musa spp.) from China using ISSR markers. Aust J Crop Sci 5:25–31
Martin KP, Pachathundikandi SK, Zhang C-L, Slater A (2006) RAPD analysis of a variant of banana (Musa sp.) cv. grande naine and its propagation via shoot tip culture. In vitro Cell Dev Biol Plant 42:188–192
Martins M, Sarmento D, Oliveira MM (2004) Genetic stability of micropropagated almond plantlets as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Mi ZW, Liu QL (2008) Plant regeneration from different explants of Lilium ‘Tiber’ and ‘Reizah 1’. Acta Hortic 766:143–148
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Pontaroli AC, Camadro EL (2005) Somaclonal variation in Asparagus officinalis plants regenerated by organogenesis from long-term callus cultures. Genet Mol Biol 28:423–430
Roh SM, Lawson RH (1988) Tissue culture in the improvement of Eustoma. HortSci 23:658
Rohlf FJ (2000) NTSYS-pc numerical taxonomy and multivariate analysis system. Version 2.1. Exeter Software, Setauket
Rout GR, Senapati SK, Aparajita S, Palai SK (2009) Studies on genetic identification and genetic fidelity of cultivated banana using ISSR markers. Plant Omics 2:250–258
Saker MM, Bekheet SA, Taha HS, Fahmy AS, Moursy HA (2000) Detection of somaclonal variations in tissue culture-derived date palm plants using isoenzyme analysis and RAPD fingerprints. Biol Plant 43:347–351
Thiruvengadam M, Yang C-H (2009) Ectopic expression of two MADS box genes from orchid (Oncidium Gower Ramsey) and lily (Lilium longiflorum) alters flower transition and formation in Eustoma grandiflorum. Plant Cell Rep 28:1463–1473
Tribulato A, Remotti PC, Löffler HJM (1997) Lily regenerative callus and cell cultures for transformation. Acta Hort 430:299–306
Yang H, Tabei Y, Kamada H, Kayamo T, Takaiwa F (1999) Detection of somaclonal variation in cultured rice cells using digoxygenin-based random amplified polymorphic DNA. Plant Cell Rep 18:520–526
Yang Y-H, Yin L-R, Ge H, Huang R-F, Xiao Q-M, Xie B-Y (2007) Overexpression of a novel member of ERF transcription factor JERF3 in lily enhances the tolerance to salt. Acta Hortic Sin 34:1485–1490
Acknowledgments
The authors wish to thank Dr. Ken Gruber and Laurie Gengenbach for their review and editing of the manuscript. This research was jointly supported by the Center of Excellence for Post-Harvest Technology and the Agricultural Research Program, North Carolina A&T State University, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Liu, X., Yang, G. Adventitious shoot regeneration of oriental lily (Lilium orientalis) and genetic stability evaluation based on ISSR marker variation. In Vitro Cell.Dev.Biol.-Plant 48, 172–179 (2012). https://doi.org/10.1007/s11627-012-9429-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9429-0