Abstract
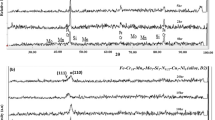
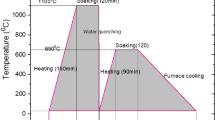
In this research, the electrochemical properties of a stir cast Al-0.65Mg-0.15Sn-0.05Ga (wt.%) alloy as an anode material in 3 wt.% NaCl solution was examined by potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). The corrosion behavior of the material was also evaluated using self-corrosion rate, hydrogen evolution, open circuit and closed circuit potentials, and anode efficiency measurements. In addition, the microstructure of the material was studied using scanning electron microscopy (SEM) and the intermetallic particles were analyzed by energy dispersive spectrometer (EDS). The results showed that tin inclusions were formed within the grains and along the grain boundaries and the dissolution of aluminum substrate was occurred preferentially around these particles leading to form round pits. The dissolution of alloy was accompanied by hydrogen gas evolution from cathodic tin particles. Polarization measurements showed active behavior with considerably negative corrosion potential value without any passive region. The obtained impedance results showed an increase in the impedance values due to the coverage of anode surface by reaction products during immersion. A sample of the alloy which was subjected to homogenizing annealing at 570 °C showed more active behavior by providing more negative open and closed circuit potential values and improved anode efficiencies at higher impressed currents, but evolved higher amounts of hydrogen compared to the as-cast anode.

This paper aims at evaluating the electrochemical and microstructural characteristics of an as-cast and solution-annealed Al-0.65Mg-0.15Sn-0.05Ga alloy anode which was fabricated by stir casting. The polarization and EIS results indicate negative corrosion potential and OCP values without passive region. The activity and anodic efficiency of the solution-annealed anode was higher in high impressed current densities but evolves more hydrogen










Similar content being viewed by others
References
Cho YJ, Park IJ, Lee HJ, Kim JG (2015) Aluminum anode for aluminum-air battery-part I: influence of aluminum purity. J Power Sources 277:370–378
Fan L, Lu H (2015) The effect of grain size on aluminum anodes for Al-air batteries in alkaline electrolytes. J Power Sources 284:409–415
Wysocka J, Krakowiak S, Ryl J, Darowicki K (2016) Investigation of the electrochemical behaviour of AA1050 aluminium alloy in aqueous alkaline solutions using dynamic electrochemical impedance spectroscopy. J Electroanal Chem 778:126–136
Dhayabaran VV, Merlin JP, Lydia IS, Shanthi R, Sivaraj R (2004) Inhibition of corrosion of aluminium in presence of fluorescein in basic medium. Ionics 10(3):288–290
Egan DR, CPd L, RJK W, Jones RL, Stokes KR, Walsh FC (2013) Developments in electrode materials and electrolytes for aluminium-air batteries. J Power Sources 236:293–310
Pino M, Chacon J, Fatas E, Ocon P (2015) Performance of commercial aluminium alloys as anodes in gelled electrolyte aluminium-air batteries. J Power Sources 299:195–201
Flamini DO, Saidman SB (2012) Electrochemical behavior of Al-Zn-Ga and Al-In-Ga alloys in chloride media. Mater Chem Phys 136:103–111
Gudic S, Smoljko I, Kliskic M (2010) The effect of small addition of tin and indium on the corrosion behavior of aluminium in chloride solution. J Alloy Compd 505:54–63
Pino M, Herranz D, Chacon J, Fatas E, Ocon P (2016) Carbon treated commercial aluminium alloys as anodes for aluminium-air batteries in sodium chloride electrolyte. J Power Sources 326:296–302
Gudic S, Radosevic J, Smoljco I, Kliskic M (2005) Cathodic breakdown of anodic oxide film on Al and Al–Sn alloys in NaCl solution. Electrochim Acta 50:5624–5632
Sharma A, Zhang C, Austin Chang Y, Knoeppel R, Morgan D (2011) Ab initio and thermodynamic modelling of alloying effects on activity of sacrificial aluminium anodes. Corros Sci 53:1724–1731
Drazic DM, Popic J (1993) Hydrogen evolution on aluminium in chloride solutions. J Electroanal Chem 357(1–2):105–116
Kliskic M, Radosevic J, Aljinovic LJ (1994) Behaviour of Al-Sn alloy on the negative side of the open-circuit potential. J Appl Electrochem 24:814–818
Venugopal A, Raja VS (1997) AC impedance study on the activation mechanism of aluminium by indium and zinc in 3.5% NaCl medium. Corros Sci 39:2053–2065
Saidman SB, Garcia SG, Bessone JB (1995) Electrochemical behavior of Al-In alloys in chloride solutions. J Appl Electrochem 25:252–258
Tuck CDS, Hunter JA, Scamans GM (1987) The electrochemical behavior of Al-Ga alloys in alkaline and neutral electrolytes. J Electrochem Soc 134(12):2970–2981
Ma J, Wen J, Li Q, Zhang Q (2013) Effects of acidity and alkalinity on corrosion behaviour of Al-Zn-Mg based anode alloy. J Power Sources 226:156–161
Despic AR, Drazic DM, Purenovic MM, Cikovic N (1976) Electrochemical properties of aluminium alloys containing indium, gallium and thallium. J Appl Electrochem 6:527–542
Barbucci A, Cerisola G, Bruzzone G, Saccone A (1997) Activation of aluminium anodes by the presence of intermetallic compounds. Electrochim Acta 42:2369–2380
Nestoridi M, Pletcher D, Wood RJK, Wang S, Jones RL, Stokes KR, Wilcock I (2008) The study of aluminium anodes for high power density Al/air batteries with brine electrolytes. J Power Sources 178:445–455
Shayeb HAE, Wahab FMA, Abedin SZE (2001) Effect of gallium ions on the electrochemical behaviour of Al, Al-Sn and Al-Zn-Sn alloys in chloride solutions. Corros Sci 43:643–654
Ma ZQ, Zuo L, Pang X, Zeng SM (2009) Effects of electrolyte components on properties of Al alloy anode. Trans Nonferrous Met Soc China 19:160–165
Ma J, Wen J (2009) The effects of lanthanum on microstructure and electrochemical properties of Al–Zn–In based sacrificial anode alloys. Corros Sci 51:2115–2119
Ma Z, Li X (2011) The study on microstructure and electrochemical properties of Al-Mg-Sn-Ga-Pb alloy anode material for Al/AgO battery. J Solid State Electrochem 15:2601–2610
Tuck CDS (1992) Use of video techniques to study the electrochemical activation and corrosion of aluminium alloys. Faraday Discuss 94:171–182
Shen PK, Tseung ACC, Kuo C (1994) Development of an aluminium/sea water battery for sub-sea applications. J Appl Electrochem 47:119–127
Budevski E, Iliev I, Kaisheva A, Despic A, Krsmanovic K (1989) Investigations of a large-capacity medium-power saline aluminium-air battery. J Appl Electrochem 19(3):323–330
Hunter JA, Scamans GM, O’Callaghan WB, Wycliffe PA (1991) Aluminium batteries. US Patent 5004654
Gao JW, Wen JB, He JG (2013) The research of electrochemical properties of Al-Mg-Sn-Ga-Bi alloys in alkaline electrolyte. Adv Mater Res 800:488–491
Ma J, Wen J, Gao J, Li Q (2014) Performance of Al-0.5 Mg-0.02 Ga-0.1 Sn-0.5 Mn as anode for Al-air battery in NaCl solutions. J Power Sources 253:419–423
Standard Guide for Laboratory Immersion Corrosion Testing of Metals (2012) ASTM NACE/ASTMG31-12a. ASTM International, West Conshohocken, PA
Impressed Current Laboratory Testing of Aluminum and Zinc Alloy Anodes (2012) NACE TM0190-2012. NACE International, Houston, TX
Graver B, Pedersen AM, Nisancioglu K (2009) Anodic activation of aluminum by trace element tin. ECS Trans 16(52):55–69
Barbucci A, Bruzzone G, Delucchi M, Panizza M, Cerisola G (2000) Breakdown of passivity of aluminium alloys by intermetallic phases in neutral chloride solution. Intermetallics 8:305–312
Murray JL (1982) The Al-Mg (aluminum-magnesium) system. Bull Alloy Phase Diagr 3(1):60–74
Jain S, Lim MLC, Hudson JL, Scully JR (2012) Spreading of intergranular corrosion on the surface of sensitized Al-4.4Mg alloys: a general finding. Corros Sci 59:136–147
Murray (1983) The Al-Ga (aluminum-gallium) system. Bull Alloy Phase Diagr 4(2):183–190
Birkin PR, Nestoridi M, Pletcher D (2009) Studies of the anodic dissolution of aluminium alloys containing tin and gallium using imaging with a high-speed camera. Electrochim Acta 54:6668–6673
Nestoridi M, Pletcher D, Wharton JA, Wood RJK (2009) Further studies of the anodic dissolution in sodium chloride electrolyte of aluminium alloys containing tin and gallium. J. Power Sources 193:895–898
Szklarska-Smialowska Z (1999) Pitting corrosion of aluminum. Corros Sci 41(9):1743–1767
Pyun S-I, Moon S-M (2000) Corrosion mechanism of pure aluminium in aqueous alkaline solution. J Solid State Electrochem 4(5):267–272
Burstein GT, Cinderey RJ (1992) Evolution of the corrosion potential of repassivating aluminium surfaces. Corros Sci 33(3):475–492
Macdonald JR (1987) Impedance spectroscopy and its use in analyzing the steady-state AC response of solid and liquid electrolytes. J Electroanal Chem Interfacial Electrochem 223(1–2):25–50
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM, El-Zayady AM, Saadawy M (2006) Kinetics and thermodynamics of aluminium dissolution in 1.0 M sulphuric acid containing chloride ions. Mater Chem Phys 98(2–3):291–297
Brug GJ, Eeden ALG, Sluyters-Rehbach M, Sluyters JH (1984) The analysis of electrode impedances complicated by the presence of a constant phase element. J Electroanal Chem Interfacial Electrochem 176(1–2):275–295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
• As-cast anode shows active anodic behavior without any passive region.
• Annealed anode exhibits improved anodic efficiencies at higher impressed currents.
• Hydrogen evolution is higher on the annealed anode surface than the as-cast anode.
• Performance of the anodes decline with time by coverage of anode with reaction products.
Rights and permissions
About this article
Cite this article
Sovizi, M.R., Afshari, M., Jafarzadeh, K. et al. Electrochemical and microstructural investigations on an as-cast and solution-annealed Al–Mg–Sn–Ga alloy as anode material in sodium chloride solution. Ionics 23, 3073–3084 (2017). https://doi.org/10.1007/s11581-017-2099-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2099-5