Abstract
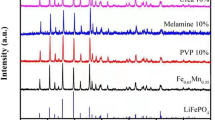
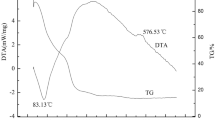
LiFePO4/C has been synthesized by polymer gel combustion method. Of aniline monomer, 0.5 and 1.0 ml were used for the different amount of gel formation. The particle sizes of LiFePO4/C composites designated as lithium iron phosphate (0.5) (LFP(0.5)) and LFP(1.0) were estimated as ∼400 and ∼100 nm for the powders synthesized with 0.5 and 1.0 ml of monomers, respectively. The final particle size of the LiFePO4/C depends on the initial monomer content used in the synthesis process. Thicknesses of carbon coating on the particles of LFP(0.5) and LFP(1.0) powders, as revealed by TEM observation, are ∼3 and ∼7 nm, respectively. The sample LFP(1.0) delivers discharge capacities of 72 and 60 mAh g−1 which are higher than those of LFP(0.5) at fast discharging rates of 5 and 10 C. The higher rate capability of sample LFP(1.0) was due to small particle size, low charge transfer resistance, and higher Li+ diffusion coefficient as compared to LFP(0.5).












Similar content being viewed by others
References
Padhi AK, Nanjundaswamy K, Goodenoug JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Andersson AS, Kalska B, Haggstrom L, Thomas JO (2000) Lithium extraction/insertion in LiFePO4: an X-ray diffraction and Mössbauer spectroscopy study. Solid State Ionics 130:41–52. doi:10.1016/S0167-2738(00)00311-8
Yang Z, Xia J, Zhi L, Zhang W, Pei B (2014) An improved solid-state reaction route to Mg2+-doped LiFePO4/C cathode material for Li-ion battery. Ionics 20:169–174. doi:10.1007/s11581-013-0974-2
Wang J, Sun X (2012) Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ Sci 5:5163–5185. doi:10.1039/C1EE01263K
Yamada A, Chung SC, Hinokuma K (2001) Optimized LiFePO4 for lithium battery cathodes. J Electrochem Soc 148(3):A224–A229. doi:10.1149/1.1348257
Wu XL, Jiang LY, Cao FF, Guo YG, Wan LJ (2009) LiFePO4 nanoparticles embedded in a nanoporous carbon matrix: superior cathode material for electrochemical energy-storage devices. Adv Mater 21:2710–2714. doi:10.1002/adma.200802998
Guo YG, Hu JS, Wan LJ (2008) Nanostructured materials for electrochemical energy conversion and storage devices. Adv Mater 20:2878–2887. doi:10.1002/adma.200800627
Xu B, Qian D, Wang Z, Meng YS (2012) Recent progress in cathode materials research for advanced lithium ion batteries. Mater Sci Eng R 73:51–65. doi:10.1016/j.mser.2012.05.003
Lv YJ, Su J, Long YF, Lv XY, Wen YX (2014) Effect of milling time on the performance of bowl-like LiFePO4/C prepared by wet milling-assisted spray drying. Ionics 20:471–478. doi:10.1007/s11581-013-1002-2
Chen WM, Qie L, Yuan LX, Xia SA, Hu XL, Zhang WX, Huang YH (2011) Insight into the improvement of rate capability and cyclability in LiFePO4/polyaniline composite cathode. Electrochim Acta 56:2689–2695. doi:10.1016/j.electacta.2010.12.041
Chena Z, Dahna JR (2002) Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density. J Electrochem Soc 149(9):A1184–A1189. doi:10.1149/1.1498255
Masquelier C, Croguennec L (2013) Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem Rev 113:6552–6591. doi:10.1021/cr3001862
Kong LB, Zhang P, Liu MC, Liu H, Luo YC, Kang L (2012) Fabrication of promising LiFePO4/C composite with a core–shell structure by a moderate in situ carbothermal reduction method. Electrochim Acta 70:19–24. doi:10.1016/j.electacta.2012.02.102
Wang YG, Wang YR, Hosono EJ, Wang KX, Zhou HS (2008) The design of a LiFePO4/Carbon nanocomposite with a core–shell structure and its synthesis by an in situ polymerization restriction method. Angew Chem Int Ed 47:7461–7465. doi:10.1002/anie.200802539
Singla ML, Sehrawat R, Rana N, Singh K (2011) Dielectric behaviour of emeraldine base polymer–ZnO nanocomposite film in the low to medium frequency. J Nanoparticle Res 13:2109–2116. doi:10.1007/s11051-010-9968-4
Kang ET, Neoh KG, Tan KL (1998) Polyaniline: a polymer with many interesting intrinsic redox states. Prog Polym Sci 23:277–324. doi:10.1016/S0079-6700(97)00030-0
Kulkarni M, Kale B, Apte S, Naik S, Mulik U, Amalnerkar D (2011) Synthesis and characterization of polyaniline nanofibres by rapid liquid-liquid interfacial polymerization method. Chem Chem Technol 5:55–58
Zaghib K, Julien CM (2005) Structure and electrochemistry of FePO4.2H2O hydrate. J Power Sources 142:279–284. doi:10.1016/j.jpowsour.2004.09.042
Wang YG, Li HQ, Xia YY (2006) Ordered whisker like polyaniline grown on the surface of mesoporous carbon and its electrochemical capacitance performance. Adv Mater 18:2619–2623. doi:10.1002/adma.200600445
Scaccic S, Carewska M, Bartolomeo AD, Prosini PP (2002) Thermoanalytical investigation of iron phosphate obtained by spontaneous precipitation from aqueous solutions. Thermochim Acta 383:145–152. doi:10.1016/S0040-6031(01)00686-4
Wilcox JD, Doeff MM, Marcinek M, Kostecki R (2007) Factors influencing the quality of carbon coatings on LiFePO4. J Electrochem Soc 154(5):A389–A395. doi:10.1149/1.2667591
Kostic R, Miric M, Radic T, Radovic M, Gajic R, Popovic ZV (2009) Optical characterization of graphene and highly oriented pyrolytic graphite. Acta Phys Pol A 116:718–721
Reich S, Thomsen C (2004) Raman spectroscopy of graphite. Phil Trans R Soc Lond A 362:2271–2288. doi:10.1098/rsta.2004.1454
Dresselhaus MS, Jorio A, Saito R (2010) Characterizing graphene, graphite, and carbon nanotubes by Raman spectroscopy. Annu Rev Condens Matter Phys 1:89–108. doi:10.1146/annurev-conmatphys-070909-103919
Vujkovic M, Stojkovic I, Cvjeticanin N, Mentus S (2003) Gel-combustion synthesis of LiFePO4/C composite with improved capacity retention in aerated aqueous electrolyte solution. Electrochim Acta 92:248–256. doi:10.1016/j.electacta.2013.01.030
Zhang H, Liu D, Qian X, Zhao C, Xu Y (2014) A novel nano structured LiFePO4/C composite as cathode for Li-ion batteries. J Power Sources 249:431–434. doi:10.1016/j.jpowsour.2013.10.109
Zhao SX, Ding H, Wang YC, Li BH, Nan CW (2013) Improving rate performance of LiFePO4 cathode materials by hybrid coating of nano-Li3PO4 and carbon. J Alloys Compd 566:206–211. doi:10.1016/j.jallcom.2013.03.041
Gao F, Tang Z (2008) Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries. Electrochim Acta 53:5071–5075. doi:10.1016/j.electacta.2007.10.069
Shu H, Wang X, Wu Q, Liu L, Liang Q, Yang S, Ju B, Yang X, Zhang X, Wang Y, Wei Q, Hu B, Liao Y, Jiang H (2012) The effect of ammonia concentration on the morphology and electrochemical properties of LiFePO4 synthesized by ammonia assisted hydrothermal route. Electrochim Acta 76:120–129. doi:10.1016/j.electacta.2012.04.156
Dominko R, Bele M, Gaberscek M, Remskar M, Hanzel D, Pejovnik S, Jamnika J (2005) Impact of the carbon coating thickness on the electrochemical performance of LiFePO4ÕC composites. J Electrochem Soc 152(3):A607–A610. doi:10.1149/1.1860492
Cho YD, Fey GTK, Kao HM (2009) The effect of carbon coating thickness on the capacity of LiFePO4/C composite cathodes. J Power Sources 189:256–262. doi:10.1016/j.jpowsour.2008.09.053
Zhang W, Hu Y, Tao X, Huang H, Gan Y, Wang C (2010) Synthesis of spherical LiFePO4/C via Ni doping. J Phys Chem Solids 71:1196–1200. doi:10.1016/j.jpcs.2010.04.015
Acknowledgments
The author Rajeev Sehrawat would like to acknowledge the financial support from the Council of Scientific Industrial and Research (CSIR), India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sehrawat, R., Sil, A. Polymer gel combustion synthesis of LiFePO4/C composite as cathode material for Li-ion battery. Ionics 21, 673–685 (2015). https://doi.org/10.1007/s11581-014-1229-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1229-6