Abstract
A sensitive electrochemical method was developed for the voltammetric determination of thymine at a composite film-modified electrode 1-phenyl-3-methyl-4-(2-furoyl)-5-pyrazolone (HPMαFP)/polypyrrole (Ppy)/glassy carbon electrode (GCE). The electrochemical parameters of thymine were investigated by cyclic voltammetry and differential pulse voltammetry. In pH = 7.4, one sensitive oxidation peak of thymine with E pa = 0.968 V was observed on the HPMαFP/PPy-modified electrode. The difference of peak potential (∆E pa) was 188 mV lower than that for bare GCE. Compared to the bare GCE and Ppy/GCE, the HPMαFP/Ppy/GCE-modified electrode showed an excellent electrocatalytic effect on the oxidation of thymine and displayed a shift of the oxidation potential in the negative direction with significant increase in the peak current. Under the optimum condition, the concentration calibration range and detection limit are 2 × 10−6–1 × 10−4 and 4.85 × 10−7 M for thymine. This developed method had been applied to the direct determination of thymine in medical pipefish samples with satisfactory results.
Similar content being viewed by others
Introduction
Pyrimidine and purine derivatives play a crucial role in various biological heritage information storage and in protein biosynthesis processes [1]. In particular, the nucleotides constituting by thymine, cytosine, adenine, and guanine represent the monomer units of deoxyribonucleic acid (DNA), whereas ribose and phosphate groups of the nucleotide units have a structure role. The abnormal changes of these bases in organisms suggest the deficiency and mutation of the immunity system and may indicate the presence of various diseases [2]. Hence, the quantitative analysis of thymine is very essential and has great significance to bioscience and clinical iatrology.
Investigations of the redox behavior of the biologically occurring compounds by means of electrochemical techniques have the potential for providing valuable insights into biological redox reactions of such biomolecules. In recent years, a series of methods have been established to indagate the content of the thymine and cytosine in samples. The electrochemical studies have been undertaken concerning their redox behavior at mercury electrode, yet thymine was reported to show no polargraphic reduction wave. In addition, the pyrimidine forms sparingly soluble compounds with Hg(II) at the surface of mercury electrode, which allows their determination by cathodic stripping voltammeters [3–6]. Due to low signal-to-noise ratio and their extreme positive oxidation potentials, thymine and cytosine have been assumed to exhibit no electrochemical activity at graphite electrodes [7, 8] or carbon-based electrodes in general [9, 10]. Brett reported oxidation of thymine and cytosine at glassy carbon electrode in alkaline pH solution after ultrasonic pretreatment [11]. However, low-stability response is the main problem when conventional carbon electrodes have been used. Furthermore, purine and pyrimidine bases adsorbed strongly at carbon electrodes [12–14], which reduced reproducibility of the analytical method. After an ultrasonic pretreatment of glassy carbon electrodes, both thymine and cytosine undergo well-defined oxidation [15].
Electropolymerization is a simple but powerful method in targeting different selective modified electrodes with desired matrices. The electroactive polymers material has received considerable attraction in recent years. Numerous conjugated polymers were electrochemically synthesized for their application in the chemical and biochemical sensor devices [16]. These polymers exhibit an interesting enhancement in the electrocatalytic activity towards the oxidation or reduction of several biochemical compounds [17], where some functional groups in the polymers act as catalyst [18–20]. The word “enhanced electrocatalytic activity” could be explained as: both increase in peak current and lower in over potential [21]. In this work, polypyrrole (Ppy) was prepared as one of the most extensively used conducting polymers [22] because of its ease of fabrication, high conductivity, good biocompatibility, and low cost [23–26]. The existence of surface positive charge on the Ppy film could provide a selective interface for the molecular interaction. The amine group (–NH–) on the pyrrole ring may lead to enhancement of biomolecular sensing [27–29]. In addition, the sensitivity of a Ppy-modified electrode could be significantly improved when it is doped with suitable active materials such as peptide [30] and DNA [31]. Nowadays, this polymer becomes one of the major tools for nanobiotechnological applications [32]. Various approaches have been considered for the synthesis of Ppy, including chemical and electrochemical methods [33]. It was reported that the catalytic activity of Ppy films depends on the synthesis conditions [33, 34].
1-Phenyl-3-methyl-4-(2-furoyl)-5-pyrazolone is an interesting class of β-diketones, containing a pyrazole fused to a chelating arm. The compounds have played an important role in including coordination chemistry and have been widely used in potential antitumor agents areas. The neutral acylpyrazolones may exist with several tautomeric forms as reported [35, 36]. The enolate form of the acylpyrazolones present weakly acidic characteristics, as a surfactant, which has practical significance in polarographic determination of trace metals and makes it suitable for electrochemical analysis [37, 38]. Due to the aromaticity, 1-phenyl-3-methyl-4-(2-furoyl)-5-pyrazolone (HPMαFP) can be adsorbed onto the surfaces of the Ppy through π–π force and hydrogen bond to form the composite film [39]. To the best of our knowledge, there are no reports on the fabrication of the HPMαFP/Ppy/GCE composite film-modified electrode and its response on thymine.
In our previous work, HPMαFP-modified GCE (HPMαFP/GCE) was applied to determination of amino acid and purine [40, 41]. In order to extend of the application of HPMαFP-modified electrode in the bioelectrochemistry, we are reporting a novel electrochemical strategy to prepare the composite film glassy carbon electrode of HPMαFP and Ppy, which shows the great capability of sensitive and selective determination of thymine.
Experimental
Apparatus
All the electrochemical measurements that contain cyclic voltammetric and differential pulse voltammetry (DPV) were performed in an analytical system model CHI-650A electrochemical workstation (Shanghai Chenhua Instrument Company, China). A conventional three-electrode system was used with a platinum wire as an auxiliary electrode and a saturated calomel electrode as reference electrode. The working electrode was either an unmodified GCE or a polymer film modified electrode (φ4 mm) for the electrochemical measurements.
Materials
Thymine was obtained from National Institute for the control of pharmaceutical and biological products (Shanghai, China, www.reagent.com.cn). A 1 × 10−2 M stock solution of thymine was done with twice distilled water in a refrigerator at approximately 277 K and remained stable for at least 1 month. Working standard solutions were prepared by suitable dilution of the stock standard solution. Pyrrole (analytical reagent, for short py, made in Shanghai reagent company, China) was made with 0.1 % water solutions.
HPMαFP was synthesized according to the method proposed by Dong [42 (yield, 73 %; mp, 373.5–374.5 K) with further purification, and a standard solution (0.01 M) of HPMαFP was prepared by anhydrous ethanol solution. Other chemicals were of analytical reagent grade and used without further purification. Of the phosphate buffer solution (PBS) of various pH values, 0.10 M was used as supporting electrolyte. All solutions were prepared with double-distilled water and experiments were conducted at the room temperature (298 ± 2 K).
Preparation of the modified electrode
Prior to modification, the bare GCE was polished successively with 1.0 and 0.3 μm alumina slurry on chamois leather, respectively. Electrode was rinsed with double-distilled water. After that, the electrode was put into nitric acid (1:1), anhydrous ethanol, in order to remove adhered alumina and rinsed thoroughly with distilled water. Glass carbon electrodes after polishing and ultrasonic cleaning were put into 0.5 M H2SO4 solution, then it was polarized to deal with cyclic voltammetry scanning, until a reproducible voltammogram was obtained (about 10 min). The electrode pretreated was put in pure water for standby. The electrode set into system: 0.1 M H3PO4–NaH2PO4 (pH = 3.0) + 0.1 M py mixed solution, connecting three electrodes system and electrochemistry workstation, controlling the scan rate of 0.25 V/s and scanning potential range of −0.1∼0.7 V. The blue–purple polymer film was obtained by cyclic scanning 20 times (the continuous cyclic voltammograms of polymerization process; Fig. 1). Ppy prepared in this conditions was very steady, the properties of Ppy has not evidently changes after putting in PBS (pH = 3.0) after 4 weeks. Finally, the GCE was carefully coated with 1 μL of HPMαFP solution and left for the solvent to evaporate at room temperature in the air. When the modified electrode completely dried, it can be used. The HPMαFP coated on the Ppy/GCE was also very steady in the PBS solution.
Experimental procedure
PBS 0.1 M (pH = 7.4) with certain amount of thymine was transferred into a cell, and the three-electrode system was installed in it. High-purity N2 was used to remove oxygen. Differential pulse voltammetry was recorded between 0.6 and 1.5 V. All experiments were carried out at room temperature (298 ± 2 K).
Preparation of medical pipefish solution
The medical pipefish (Syngnathus acus; Haikou, China) was ground into powder in agate mortar. The sample powder (1.450 g) was weighed and extracted with 25 mL double-distilled water by ultrasonic wave for 90 min. The clear treated solution (10 mL) was transferred to a 25 mL volumetric flask and diluted to the mark with double-distilled water as stock for the experiments.
Results and discussion
The microstructure of the modified electrode
The microscopic structure of modified electrodes was characterized using scanning electron microscope images. Figure 2 exhibits typical images of polypyrrole, HPMαFP, and HPMαFP/Ppy films coated onto GCE. As it can be clearly seen, Ppy-modified electrode (Fig. 2a) surface shows a uniform formation and arranged flakes of polymer layers. The surface area of the modified electrode was substantially increases. HPMαFP (Fig. 2b) was dispersed over the glassy carbon surface, and HPMαFP adsorbed on the surface of Ppy at the HPMαFP/Ppy film (Fig. 2c). The reasons were concluded in the following aspects: (1) At the polypyrrole and HPMαFP, the active functional groups maybe existed, such as –NH–, carbonyl, and hydroxy. The intermolecular effect (hydrogen bond) was formed between Ppy and HPMαFP; (2) Some aromaticities was consistent in HPMαFP containing benzene, parazole, and furfuran. The π–π interactions between the aromaticity of HPMαFP and pentagonal structure of Ppy were attributed to the easy arrival of HPMαFP to the surface of Ppy [39] (as shown in Scheme 1).
Electrochemical behavior of the modified electrode
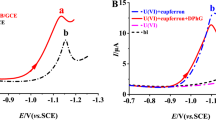
The differential pulse voltammograms of thymine at a bare GCE-, Ppy/GCE-, and HPMαFP/Ppy-modified GC electrode in phosphate buffer (pH = 7.4) were shown in Fig. 3. It can be seen that the thymine oxidation peak at the bare GCE and Ppy/GCE was weak and broad due to slow electron transfer, while the response was considerably improved at the HPMαFP/Ppy/GCE. The peak potential at above three electrodes were at defferent position, at the bare GCE, an irreversible oxidation peak appeared at about 1.156 V (Fig. 3a). At the Ppy/GCE, the oxidation peak is located at 0.984 V (Fig. 3b). The oxidation peak potential (E pa) was negatively shifted for 0.172 V and the oxidation peak current (I pa) increased clearly. Both the oxidation peak current and potential of thymine were increased and moved negatively 0.968 V slightly at HPMαFP/Ppy/GCE-modified electrode (Fig. 3c).
From the comparison of voltammograms of thymine, it can be observed that the peak current at HPMαFP/Ppy/GCE was more than two times higher than the sum of response currents at bare GCE or Ppy/GCE. The above results indicated that the composite film-modified electrode exhibited the synergistic effect including high conductivity, fast electron transfer rate, and inherent catalytic ability [43]. This phenomenon was probably due to HPMaFP contained some oxygen functional groups such as carbonyl, which was propitious to thymine oxidation, these oxygen functional groups could form hydrogen bonds with the amidogen of thymine. Moreover, the hydrogen bond maybe existed between Ppy and HPMαFP or thymine and Ppy, which can remarkably increase the accumulation amount of thymine (as shown in Scheme 2).
Effect of Ppy–HPMaFP amount
The relationship between the amount of Ppy–HPMαFP dispersion on the GCE surface and the oxidation peak current of thymine was investigated. The results showed that the variation of oxidation peak current of thymine was as a function of the amount of Ppy–HPMαFP (Fig. 4). When the amount of Ppy increased from 10 to 20 times, the oxidation peak current increased notably (Fig. 4a). However, the oxidation peak current showed conversely gradual decline when the amount of Ppy exceeds 20 times. The reason is that too thick polypyrrole film may be more easily abraded from the surface of GCE. So, the polymerization scanning polypyrrole intercalative 20 times was selected. On the other hand, the oxidation peak current gradually increased with the augment of quantity of HPMαFP. The results were shown in Fig. 4b. When the HPMαFP was beyond 1.0 μL, the peak current decreased. This was due to the surface of Ppy/GCE casted too thick to impede the charge exchange. To sum up, 20 times polypyrrole and 1.0 μL HPMαFP was selected in the subsequent analytical experiments and the maximum electrocatalytic response on thymine was achieved.
Effect of solution pH
Influence of pH on electrochemical behavior of 1.0 × 10−5 M thymine at HPMαFP/Ppy/GCE was studied in the pH range from 5.0 up to 8.0. Both the peak current and potentials were depended on pH values of solution. The results were shown in Fig. 5. With the increment of pH, the oxidation peak current of thymine at the HPMαFP/Ppy/GCE-modified electrode decreased slightly and the peak potential was found to shift negatively. The pH values around 7.4 should provide the highest oxidation currents justifying the selection of pH 7.4 for the subsequent experiments using the HPMαFP/Ppy/GCE film electrode. According to the equation: −59χ/n = −61, where χ is the hydroxyl ion participating the electrode reaction and n is the electron-transfer number, the slope of the E pa–pH is 61 mV. The number of OH-s and transferred electrons are 1. So, the loss of electrons was accompanied by the loss of an equal amount of hydroxyl ion and χ = n = 1. The reaction mechanism for thymine was shown in Scheme 3 [44].
Effect of sweep rate
The effect of scan rate on the voltammetric response for the oxidation of 1.0 × 10−4 M thymine on the HPMαFP/Ppy/GCE was investigated from 30 to 350 mV s−1. The cyclic voltammetric results demonstrated that the peak current varied linearly with the square root of the scan rate according to the equation: I pa(A) = −1.923 + 1.333v1/2, with an excellent linear correlation coefficient of 0.9990. Such behaviors indicating that the HPMαFP/Ppy composites could efficiently accelerate the electron transfer at the GCE with a diffusion-controlled process (Fig. 6).
Determination of thymine
DPV technique was employed to develop a voltammetric method for determination of thymine. Under the optimal conditions, voltammograms at these concentrations were shown in Fig. 7a, and two linear calibration graphs were obtained (Fig. 7b). The initial linear portion increased from 2 × 10−6–1 × 10−5 M with a linear regression equation of I pa(A) = 0.175C + 0.019 (r = 0.9984). The second linear segment is from 1 × 10−5 M up to 1 × 10−4 M with a linear regression equation of I pa(A) = 0.049C + 1.391 (r = 0.9994). The detection limit was found to be 4.85 × 10−7 M. This detection limit was lower, and the sensitivities were higher than those obtained by other electrochemical methods.
Determination of thymine in the presence of xanthine
The main objective of the present study is to selectively determine thymine in the presence of xanthine. Figure 8 showed the DPVs for the increment of 2 μM thymine in the presence of 200 μM xanthine in 0.1 M PBS (pH = 3.0). During the anodic sweep from 0.1 to 1.2 V, two oxidation peaks at 0.687 V (xanthine) and 0.968 V (thymine) were observed at the HPMαFP/Ppy/GCE. The oxidation current of thymine was linearly decreases by its concentration from 2 to 20 μM with a correlation coefficient of r = 0.9996(I pa(μA) = 15.39998C–27.45901). These results indicated that HPMαFP/Ppy/GCE was highly selective towards the oxidation of thymine and detection of very low concentration thymine was possible in the presence of an excess of xanthine.
Stability and repeatability of the modified electrode
In order to investigate the repeatability of HPMαFP/Ppy/GCE, the differential pulse voltammetry values for 1 × 10-4 M thymine were recorded in 6-min intervals. It was shown that the peak of thymine nearly remained the same, and the relative standard (RSD) gave the result of 1.6 %, indicating excellent repeatability of the modified electrode.
The stability of the modified electrode was also investigated over a period of 7 days. The modified electrode was used daily and stored in air. The peak potential for thymine was unchanged, and the current signals showed only less than 3 % decrease of the initial response, which suggested that the HPMαFP/Ppy/GCE possessed good stability for the detection of adenine.
Interferences
The effects of interfering ions or biomolecules on the determination of thymine were investigated. Under experimental optimal conditions, the proposed method was used for the determination of thymine at 1 × 10−4 M level, some interference experiments were carried out. The tolerance limit was taken as approximately 5 % relative error of the foreign substance. The experimental results showed that at least a 200-fold excess of Na+, K+, Cl−, H2PO4 −, HPO4 2−, PO4 3−, a 100-fold excess of DL–serine, l-leucine, l-lysine, a 50-fold excess of cytosine, glucose, lysine, and a 20-fold excess of DL–phenylalanine, citric acid did not cause any observable interference for the determination of thymine.
Analysis of thymine in medical pipefish samples
The HPMαFP/Ppy/GCE was used to detect thymine in the medical pipefish (S. acus) samples. The preparative procedures for the S. acus solution were followed as described in the “Experimental” section. The determination of thymine concentration was performed by the standard addition method. The results were summarized in Table 1 with the recovery and RSD respectively from 99.2 to 100.7 and 1.1 to 1.9. The data showed that the proposed method can be used efficiently for the detection of thymine in medical samples.
Conclusions
In this work, an easily prepared HPMαFP/Ppy composite film-modified electrode was firstly performed to investigate the electrochemical behavior of thymine in detail. The electrode reaction was a diffusion-controlled irreversible process with one electrons and one hydroxyl ion. The HPMαFP/Ppy/GCE exhibited high electrocatalytic activities towards the oxidation of thymine by significantly decreasing their oxidation overpotentials and enhancing the peak currents, and used for the determination of thymine in the presence of high concentration of xanthine by DPV. Also, the proposed method offers important advantages such as easy fabrication, reproducibility, stability, and antijamming, which could be applied to the determination of thymine in medical pipefish samples with satisfactory results.
References
Shen Q, Wang XM (2009) J Electroanal Chem 632:149
Liu HY, Wang GF, Hu JS, Chen DL, Zhang W, Fang B (2008) J Appl Polym Sci 107:3173
Palecek E, Jank B (1962) Arch Biochem Biophys 98:527
Jank B, Palecek E (1964) Arch Biochem Biophys 105:225
Dryhurst G, EIving PJ (1969) Talanta 16:855
Palecek E (1966) J Mol Biol 20:263
Smith D, Elving PJ (1962) Anal Chem 8:930
Brabec V (1981) Bioelectrochem Bioenerg 8:437
Hart JP (1990) Electroanalysis of biologically important compounds. Horwood, Chichester, 46
Stul K, Pacfikovfi V (1987) Electroanalytical measurements in flowing liquids. Horwood, Chichester, 262
Brett AMO, Piedade JAP, Silva LA, Diculescu VC (2004) Anal Biochem 332:321
Yano T, Tryk DA, Hashimota K, Fujishima A (1998) J Electrochem Soc 143:1870
Wang HS, Ju HX, Chen HY (2001) Electroanalysis 13:1105
Rao TN, Sarada BV, Tryk DA, Fujishima A (2000) J Electroanal Chem 491:175
Brett AMO, Matysik FM (1997) J Electroanal Chem 429:95
McMahon CP, Rocchitta G, Kirwan SM, Killoran SJ, Serra PA, Lowry JP, O’Neill RD (2007) Biosens Bioelectron 22:1466
Becerik I, Kadirgan F (2001) Synth Met 124:379
Selvaraju T, Ramaraj RR (2005) Electroanal J Chem 585:290
Mao M, Zhang D, Sotomura T, Nakatsu K, Koshiba N, Ohsaka T (2003) Electrochim Acta 48:1015
Yasuzawa M, Kunugi A (1999) Electrochem Commun 1:459
Andrieux CP, Haas O, SavGant JM (1986) J Am Chem Soc 108:8175
Adeloju SB, Wallance GG (1996) Analyst 121:699
Hepel M (1998) J Electrochem Soc 145:124
Kim DH, Richardson-Burns SM, Hendricks JL, Sequera C, Martin DC (2007) Adv Funct Mater 17:79
Malitesta C, Palmisano F, Torsi L, Zambonin PG (1990) Anal Chem 62:2735
Pernaut JM, Reynolds JR (2000) J Phys Chem B 104:4080
Ulubay S, Dursun Z (2010) Talanta 80:1461
Li J, Lin XQ (2007) Sensors Actuators B 124:486
Li J, Lin XQ (2007) Anal Chim Acta 596:222
Cui XY, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC (2001) J Biomed Mater Res Part A 56:261
Li J, Wei W, Luo S (2010) Microchim Acta 171:109
Malinauskas A, Malinauskiene J, Ramanavicius A (2005) Nanotechnology 16:51
Schmidt HL, Gutberlet F, Schuhmann W (1993) Sensors Actuators B 13:366
Ramanaviciene A, Ramanavicius A, Thomas DW (2004) Advance biomaterials for medical applications. Kluwer, Dordrecht, p 111
Nishihama S, Hirai T, Komasawa I (2001) Ind Eng Chem Res 40:3085
Marchetti F, Pettinari C, Pettinari R (2005) Coord Chem Rev 249:2909
Ghoneim MM, El-Desoky HS, Amer SA, Rizk HF, Habazy AD (2008) Dyes Pigments 77:493
Zhang D, Li JZ (2008) Anal Lett 41:2832
Li FH, Chai J, Yang HF, Han DX, Niu L (2010) Talanta 81:1063
Li XL, Li JZ (2011) Rev Anal Chem 30:23
Song Y, Li JZ (2011) Instrum Sci Technol 39:261
Dong XC, Liu FC, Zhao YL (1983) Acta Chim Sinica 41:848
Song Y, Li JZ (2011) J Solid State Electrochem 16:693
Wu SG, Zheng LZ, Rui L, Lin XQ (2001) Electroanal 13:967
Acknowledgments
This work was supported by grants from the Nature Science Foundation of Heilongjiang Province, People's Republic of China (no. B201004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Han, H., Li, JZ., Li, Y. et al. Electrochemical methods for determination of thymine in medical pipefish samples based on HPMαFP/Ppy/GCE-modified electrode. Ionics 19, 989–996 (2013). https://doi.org/10.1007/s11581-013-0880-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0880-7