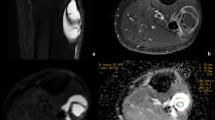
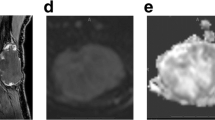
Abstract
In the last two decades, relevant progress has been made in the diagnosis of musculoskeletal tumors due to the development of new imaging tools, such as diffusion-weighted imaging, diffusion kurtosis imaging, magnetic resonance spectroscopy, and diffusion tensor imaging. Another important role has been played by the development of artificial intelligence software based on complex algorithms, which employ computing power in the detection of specific tumor types. The aim of this article is to report the most advanced imaging techniques focusing on their advantages in clinical practice.




Similar content being viewed by others
References
Franchi A (2012) Epidemiology and classification of bone tumors. Clin Cases Miner Bone Metab 9:92–95
Bruno F, Arrigoni F, Mariani S et al (2019) Advanced magnetic resonance imaging (MRI) of soft tissue tumors: techniques and applications. Radiol Medica 124:243–252
Stiller CA, Trama A, Serraino D et al (2013) Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer 49:684–695. https://doi.org/10.1016/j.ejca.2012.09.011
Verstraete KL, Lang P (2000) Bone and soft tissue tumors: The role of contrast agents for MR imaging. Eur J Radiol 34:229–246. https://doi.org/10.1016/S0720-048X(00)00202-3
Costa FM, Ferreira EC, Vianna EM (2011) Diffusion-weighted magnetic resonance imaging for the evaluation of musculoskeletal tumors. Magn Reson Imag Clin N Am 19:159–180
Robba T, Chianca V, Albano D et al (2017) Diffusion-weighted imaging for the cellularity assessment and matrix characterization of soft tissue tumour. Radiol Med 122:871–879. https://doi.org/10.1007/s11547-017-0787-x
Suzuki K (2012) Pixel-based machine learning in medical imaging. Int J Biomed Imag 2012:792079. https://doi.org/10.1155/2012/792079
Van Rijswijk CSP, Kunz P, Hogendoorn PCW et al (2002) Diffusion-weighted MRI in the characterization of soft-tissue tumors. J Magn Reson Imag 15:302–307. https://doi.org/10.1002/jmri.10061
Malayeri AA, Riham •, Khouli H El, et al (2011) Multisystem imaging principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up 1 from the. RadioGraphics 31:1773–1791 . doi: https://doi.org/10.1148/rg.316115515
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. Am J Roentgenol 188:1622–1635. https://doi.org/10.2214/AJR.06.1403
Messina C, Bignone R, Bruno A et al (2020) Diffusion-weighted imaging in oncology: an update. Cancers (Basel) 12:1493. https://doi.org/10.3390/cancers12061493
Tang L, Zhou XJ (2019) Diffusion MRI of cancer: from low to high b-values. J Magn Reson Imaging 49:23–40
Bellelli A, Silvestri E, Barile A et al (2019) Position paper on magnetic resonance imaging protocols in the musculoskeletal system (excluding the spine) by the Italian College of Musculoskeletal Radiology. Radiol Med 124:522–538. https://doi.org/10.1007/s11547-019-00992-3
Subhawong TK, Jacobs MA, Fayad LM (2014) Insights into quantitative diffusion-weighted MRI for musculoskeletal tumor imaging. Am J Roentgenol 203:560–572
Bhojwani N, Szpakowski P, Partovi S et al (2015) Diffusion-weighted imaging in musculoskeletal radiology-clinical applications and future directions. Quant Imag Med Surg 5:740–753. https://doi.org/10.3978/j.issn.2223-4292.2015.07.07
Fukuda T, Wengler K, de Carvalho R et al (2019) MRI biomarkers in osseous tumors. J Magn Reson Imag 50:702–718. https://doi.org/10.1002/jmri.26672
Wang T, Wu X, Cui Y et al (2014) Role of apparent diffusion coefficients with diffusion-weighted magnetic resonance imaging in differentiating between benign and malignant bone tumors. World J Surg Oncol. https://doi.org/10.1186/1477-7819-12-365
Yakushiji T, Oka K, Sato H et al (2009) Characterization of chondroblastic osteosarcoma: gadolinium-enhanced versus diffusion-weighted MR imaging. J Magn Reson Imag 29:895–900. https://doi.org/10.1002/jmri.21703
Savarino E, Chianca V, Bodini G et al (2017) Gadolinium accumulation after contrast-enhanced magnetic resonance imaging: Which implications in patients with Crohn’s disease? Dig Liver Dis. https://doi.org/10.1016/j.dld.2017.04.010
Doniselli FM, Albano D, Chianca V et al (2017) Gadolinium accumulation after contrast-enhanced magnetic resonance imaging: what rheumatologists should know. Clin Rheumatol 36:977–980. https://doi.org/10.1007/s10067-017-3604-y
Douis H, Jeys L, Grimer R et al (2015) Is there a role for diffusion-weighted MRI (DWI) in the diagnosis of central cartilage tumors? Skeletal Radiol 44:963–969. https://doi.org/10.1007/s00256-015-2123-7
Pozzi G, Albano D, Messina C et al (2018) Solid bone tumors of the spine: diagnostic performance of apparent diffusion coefficient measured using diffusion-weighted MRI using histology as a reference standard. J Magn Reson Imag 47:1034–1042. https://doi.org/10.1002/jmri.25826
Luo Z, Litao L, Gu S et al (2016) Standard-b-value vs low-b-value DWI for differentiation of benign and malignant vertebral fractures: a meta-analysis. Br J Radiol. https://doi.org/10.1259/bjr.20150384
Vilanova JC, Baleato-Gonzalez S, Romero MJ et al (2016) Assessment of musculoskeletal malignancies with functional MR imaging. Magn Reson Imag Clin N Am 24:239–259
Yao K, Troupis JM (2016) Diffusion-weighted imaging and the skeletal system: a literature review. Clin Radiol 71:1071–1082
Pekcevik Y, Kahya MO, Kaya A (2015) Characterization of soft tissue tumors by diffusion-weighted imaging. Iran J Radiol 12:15478. https://doi.org/10.5812/iranjradiol.15478v2
Lee SY, Jee WH, Jung JY et al (2016) Differentiation of malignant from benign soft tissue tumours: use of additive qualitative and quantitative diffusion-weighted MR imaging to standard MR imaging at 3.0 T. Eur Radiol 26:743–754. https://doi.org/10.1007/s00330-015-3878-x
Razek A, Nada N, Ghaniem M, Elkhamary S (2012) Assessment of soft tissue tumours of the extremities with diffusion echoplanar MR imaging. Radiol Medica 117:96–101. https://doi.org/10.1007/s11547-011-0709-2
Chhabra A, Ashikyan O, Slepicka C et al (2019) Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur Radiol 29:4485–4494. https://doi.org/10.1007/s00330-018-5845-9
Lee JH, Yoon YC, Seo SW et al (2020) Soft tissue sarcoma: DWI and DCE-MRI parameters correlate with Ki-67 labeling index. Eur Radiol 30:914–924. https://doi.org/10.1007/s00330-019-06445-9
Choi YJ, Lee IS, Song YS et al (2019) Diagnostic performance of diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) MRI for the differentiation of benign from malignant soft-tissue tumors. J Magn Reson Imag 50:798–809. https://doi.org/10.1002/jmri.26607
Mazal AT, Ashikyan O, Cheng J et al (2019) Diffusion-weighted imaging and diffusion tensor imaging as adjuncts to conventional MRI for the diagnosis and management of peripheral nerve sheath tumors: current perspectives and future directions. Eur Radiol 29:4123–4132
Pasoglou V, Michoux N, Larbi A, Van Nieuwenhove S, Lecouvet F (2018) Whole Body MRI and oncology: recent major advances. Br J Radiol 91:20170664. https://doi.org/10.1259/bjr.20170664
Jacobs MA, Pan L, Macura KJ (2009) Whole-body diffusion-weighted and proton imaging: a review of this emerging technology for monitoring metastatic cancer. Semin Roentgenol 44:111–122. https://doi.org/10.1053/j.ro.2009.01.003
Galia M, Albano D, Narese D et al (2016) Whole-body MRI in patients with lymphoma: collateral findings. Radiol Medica. https://doi.org/10.1007/s11547-016-0658-x
Goudarzi B, Kishimoto R, Komatsu S et al (2010) Detection of bone metastases using diffusion weighted magnetic resonance imaging: comparison with 11C-methionine PET and bone scintigraphy. Magn Reson Imag 28:372–379. https://doi.org/10.1016/j.mri.2009.12.008
Wu LM, Gu HY, Zheng J et al (2011) Diagnostic value of whole-body magnetic resonance imaging for bone metastases: a systematic review and meta-analysis. J Magn Reson Imag 34:128–135. https://doi.org/10.1002/jmri.22608
Stecco A, Trisoglio A, Soligo E et al (2018) Whole-body MRI with diffusion-weighted imaging in bone metastases: a narrative review. Diagnostics 8:45. https://doi.org/10.3390/diagnostics8030045
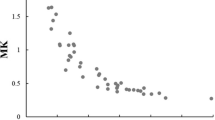
Marrale M, Collura G, Brai M et al (2016) Physics, techniques and review of neuroradiological applications of diffusion kurtosis imaging (DKI). Clin Neuroradiol 26:391–403
Wu G, Liu X, Xiong Y et al (2018) Intravoxel incoherent motion and diffusion kurtosis imaging for discriminating soft tissue sarcoma from vascular anomalies. Med (United States). https://doi.org/10.1097/MD.0000000000013641
Ogawa M, Kan H, Arai N et al (2019) Differentiation between malignant and benign musculoskeletal tumors using diffusion kurtosis imaging. Skeletal Radiol 48:285–292. https://doi.org/10.1007/s00256-018-2946-0
Cotten A, Haddad F, Hayek G et al (2015) Tractography: possible applications in musculoskeletal radiology. Semin Musculoskelet Radiol. https://doi.org/10.1055/s-0035-1563736
Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. https://doi.org/10.3389/fnins.2013.00031
Guggenberger R, Eppenberger P, Markovic D (2012) MR neurography of themedian nerve at 3.0T: optimization of diffusion tensor imaging and fiber tractography. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2012.03.017
Schlaffke L, Rehmann R, Froeling M et al (2017) Diffusion tensor imaging of the human calf : variation of inter- and intramuscle-specific diffusion parameters. J Magn Reson. https://doi.org/10.1002/jmri.25650
Qin W, Yu CS, Zhang F et al (2009) Effects of echo time on diffusion quantification of brain white matter at 1.5 T and 3.0 T. Magn Reson Med. https://doi.org/10.1002/mrm.21920
Chianca V, Albano D, Messina C et al (2017) Diffusion tensor imaging in the musculoskeletal and peripheral nerve systems: from experimental to clinical applications. Eur Radiol Exp 1:12. https://doi.org/10.1186/s41747-017-0018-1
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. https://doi.org/10.1016/j.nurt.2007.05.011
Alexander AL, Lee JE, Wu YC, Field AS (2006) Comparison of diffusion tensor imaging measurements at 3.0 T versus 1.5 T with and without parallel imaging. Neuroimag Clin N Am 16:299–309
Vetrano IG, Sconfienza LM, Albano D et al (2019) Recurrence of carpal tunnel syndrome in isolated non-syndromic macrodactyly: DTI examination of a giant median nerve. Skeletal Radiol 48:989–993. https://doi.org/10.1007/s00256-018-3098-y
Savardekar AR, Patra DP, Thakur JD et al (2018) Preoperative diffusion tensor imaging-fiber tracking for facial nerve identification in vestibular schwannoma: a systematic review on its evolution and current status with a pooled data analysis of surgical concordance rates. Neurosurg Focus. https://doi.org/10.3171/2017.12.FOCUS17672
Chhabra A, Thakkar RS, Andreisek G (2013) Anatomic MR imaging and functional diffusion tensor imaging of peripheral nerve tumors and tumorlike conditions. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A3316
Cage TA, Yuh EL, Hou SW et al (2015) Visualization of nerve fibers and their relationship to peripheral nerve tumors by diffusion tensor imaging. Neurosurg Focus. https://doi.org/10.3171/2015.6.FOCUS15235
Van Der Graaf M (2010) In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J 39:527–540
Liu Y, Gu Y, Yu X (2017) Assessing tissue metabolism by phosphorous-31 Magnetic resonance spectroscopy and imaging: a methodology review. Quant Imag Med Surg 7:707–726
Subhawong TK, Wang X, Durand DJ et al (2012) Proton MR spectroscopy in metabolic assessment of musculoskeletal lesions. Am J Roentgenol 198:162–172. https://doi.org/10.2214/AJR.11.6505
Deshmukh S, Subhawong T, Carrino JA, Fayad L (2014) Role of MR spectroscopy in musculoskeletal imaging. Indian J Radiol Imag 24:210–216. https://doi.org/10.4103/0971-3026.137024
Faghihi R, Zeinali-Rafsanjani B, Mosleh-Shirazi MA et al (2017) Magnetic resonance spectroscopy and its clinical applications: a review. J Med Imag Radiat Sci 48:233–253
Ogg RJ, Kingsley PB, Taylor JS (1994) WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson Ser B 104:1–10. https://doi.org/10.1006/jmrb.1994.1048
Amar M, Ghasi RG, Krishna LG, Khanna G (2019) Proton MR spectroscopy in characterization of focal bone lesions of peripheral skeleton. Egypt J Radiol Nucl Med 50:91. https://doi.org/10.1186/s43055-019-0109-5
Wang CK, Li CW, Hsieh TJ et al (2004) Characterization of bone and soft-tissue tumors with in vivo 1H MR spectroscopy: initial results. Radiology 232:599–605. https://doi.org/10.1148/radiol.2322031441
Doganay S, Altinok T, Alkan A et al (2011) The role of MRS in the differentiation of benign and malignant soft tissue and bone tumors. Eur J Radiol 79:e33–e37. https://doi.org/10.1016/j.ejrad.2010.12.089
Costa FM, Canella C, Gasparetto E (2011) Advanced magnetic resonance imaging techniques in the evaluation of musculoskeletal tumors. Radiol Clin North Am 49:1325–1358
Gondim Teixeira PA, Ledrich M, Kauffmann F et al (2017) Qualitative 3-T proton MR spectroscopy for the characterization of musculoskeletal neoplasms: update on diagnostic performance and indications. Am J Roentgenol 208:1312–1319. https://doi.org/10.2214/AJR.16.17285
Xu W, Hao D, Hou F et al (2020) Soft tissue sarcoma: preoperative MRI-based radiomics and machine learning may be accurate predictors of histopathologic grade. Am J Roentgenol 215:963–969. https://doi.org/10.2214/AJR.19.22147
Cabitza F, Campagner A, Albano D et al (2020) The elephant in the machine: proposing a new metric of data reliability and its application to a medical case to assess classification reliability. Appl Sci 10:4014. https://doi.org/10.3390/app10114014
Gorelik N, Gyftopoulos S (2021) Applications of artificial intelligence in musculoskeletal imaging: from the request to the report. Can Assoc Radiol J 72:45–59
Campagner A, Sconfienza L, Cabitza F (2020) H-Accuracy, an alternative metric to assess classification models in medicine. In: Studies in health technology and informatics. IOS, 16;270:242–246. https://doi.org/10.3233/SHTI200159
Erickson BJ, Korfiatis P, Akkus Z, Kline TL (2017) Machine learning for medical imaging. Radiographics 37:505–515. https://doi.org/10.1148/rg.2017160130
Chartrand G, Cheng PM, Vorontsov E et al (2017) Deep learning: a primer for radiologists. Radiographics 37:2113–2131. https://doi.org/10.1148/rg.2017170077
Do S, Song KD, Chung JW (2020) Basics of deep learning: a radiologist’s guide to understanding published radiology articles on deep learning. Korean J Radiol 21:33–41
Pesapane F, Codari M, Sardanelli F (2018) Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp 24(2):35. https://doi.org/10.1186/s41747-018-0061-6
Crombé A, Marcellin PJ, Buy X et al (2019) Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology 291:710–721. https://doi.org/10.1148/radiol.2019181659
Corino VDA, Montin E, Messina A et al (2018) Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions. J Magn Reson Imag 47:829–840. https://doi.org/10.1002/jmri.25791
Zhang Y, Zhu Y, Shi X et al (2019) Soft tissue sarcomas: preoperative predictive histopathological grading based on radiomics of MRI. Acad Radiol 26:1262–1268. https://doi.org/10.1016/j.acra.2018.09.025
Peeken JC, Spraker MB, Knebel C et al (2019) Tumor grading of soft tissue sarcomas using MRI-based radiomics. EBioMedicine 48:332–340. https://doi.org/10.1016/j.ebiom.2019.08.059
Vos M, Starmans MPA, Timbergen MJM et al (2019) Radiomics approach to distinguish between well differentiated liposarcomas and lipomas on MRI. Br J Surg 106:1800–1809. https://doi.org/10.1002/bjs.11410
Lisson CS, Lisson CG, Flosdorf K et al (2018) Diagnostic value of MRI-based 3D texture analysis for tissue characterisation and discrimination of low-grade chondrosarcoma from enchondroma: a pilot study. Eur Radiol 28:468–477. https://doi.org/10.1007/s00330-017-5014-6
Fritz B, Müller DA, Sutter R et al (2018) Magnetic resonance imaging-based grading of cartilaginous bone tumors: added value of quantitative texture analysis. Invest Radiol 53:663–672. https://doi.org/10.1097/RLI.0000000000000486
Gitto S, Cuocolo R, Albano D et al (2020) MRI radiomics-based machine-learning classification of bone chondrosarcoma. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2020.109043
Lang N, Zhang Y, Zhang E et al (2019) Differentiation of spinal metastases originated from lung and other cancers using radiomics and deep learning based on DCE-MRI. Magn Reson Imag 64:4–12. https://doi.org/10.1016/j.mri.2019.02.013
Filograna L, Lenkowicz J, Cellini F et al (2019) Identification of the most significant magnetic resonance imaging (MRI) radiomic features in oncological patients with vertebral bone marrow metastatic disease: a feasibility study. Radiol Medica 124:50–57. https://doi.org/10.1007/s11547-018-0935-y
Lin P, Yang PF, Chen S et al (2020) A Delta-radiomics model for preoperative evaluation of Neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imag 20:7. https://doi.org/10.1186/s40644-019-0283-8
Zhao S, Su Y, Duan J et al (2019) Radiomics signature extracted from diffusion-weighted magnetic resonance imaging predicts outcomes in osteosarcoma. J Bone Oncol 19:100263. https://doi.org/10.1016/j.jbo.2019.100263
Wu Y, Xu L, Yang P et al (2018) Survival prediction in high-grade osteosarcoma using radiomics of diagnostic computed tomography. EBioMedicine 34:27–34. https://doi.org/10.1016/j.ebiom.2018.07.006
Chianca V, Cuocolo R, Gitto S et al (2021) Radiomic machine learning classifiers in spine bone tumors: a multi-software. Multi-Scanner Study Eur J Radiol. https://doi.org/10.1016/j.ejrad.2021.109586
Funding
No funds, grants, or other support was received. The authors declare they have no financial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was not necessary for this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chianca, V., Albano, D., Messina, C. et al. An update in musculoskeletal tumors: from quantitative imaging to radiomics. Radiol med 126, 1095–1105 (2021). https://doi.org/10.1007/s11547-021-01368-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-021-01368-2