Abstract
Aim
To evaluate proven soft tissue musculoskeletal malignancies blinded to their Fédération Nationale des Centres de Lutte Contre le Cancer histologic grades to identify the predictive values of conventional MR findings and best fit region of interest (ROI) apparent diffusion coefficient (ADC) measurements.
Materials and methods
Fifty-one consecutive patients with different histologic grades were evaluated by four readers (R1–4) of different experience levels. Quantitatively, the maximum longitudinal size, tumor to muscle signal intensity ratios, and ADC measurements and, qualitatively, the spatial location of the tumor, its signal alterations, heterogeneity, intralesional hemorrhage or fat, and types of enhancement were assessed. Intraclass correlation, weighted kappa, ANOVA, and Fisher exact tests were used.
Results
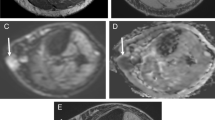
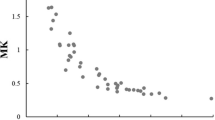
There were 22/51 (43%) men (mean age ± SD = 52 ± 16 years) and 29/51 (57%) women (mean age ± SD = 54± 17 years), with the majority of tumors 38/51 (75%) in the lower extremities. Histologic grades were I in 8/51 (16%), II in 17/51 (33%), and III in 26/51 (51%), respectively. The longitudinal dimensions were different among three grades (p = 0.0015), largest with grade I. More central enhancements and deep locations were seen in grade III tumors (p = 0.0191, 0.0246). The ADC mean was significantly lower in grade III than in grade I or II (p < 0.0001 and p = 0.04). The ADC min was significantly lower in grade III than in grade I (p = 0.02). Good to excellent agreements were seen for T1/T2 tumor/muscle ratios, longitudinal dimension, and ADC (ICC = 0.60–0.98).
Conclusion
Longitudinal tumor dimension, central enhancement, and ADC values differentiate histology grades in musculoskeletal soft tissue malignancy with good to excellent inter-reader reliability.
Key Points
• The longitudinal tumor dimension of grade III malignancy is smaller than that of grade I (p < 0.0001), and higher-grade tumors are located deeper (p = 0.0246).
• The ADC mean is significantly lower in grade III than in grade I or grade II (p < 0.0001 and p = 0.04).
• The ADC minimum is significantly lower in grade III than in grade I (p = 0.02).




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- FNCLCC:
-
Fédération Nationale des Centres de Lutte Contre le Cancer
- ROI:
-
Region of interest
References
Murphey MD, Kransdorf MJ, Smith SE (1999) Imaging of soft tissue neoplasms in the adult: malignant tumors. Semin Musculoskelet Radiol 3:39–58. https://doi.org/10.1055/s-2008-1080050
Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT (2002) Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology 224:99–104. https://doi.org/10.1148/radiol.2241011113
Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA (2006) From the archives of the AFIP: imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics 26:1543–1565. https://doi.org/10.1148/rg.265065084
Walker L, Thompson D, Easton D et al (2006) A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer 95:233–238. https://doi.org/10.1038/sj.bjc.6603227
Coran A, Ortolan P, Attar S et al (2017) Magnetic resonance imaging assessment of lipomatous soft-tissue tumors. In Vivo 31:387–395
Guillou L, Coindre JM, Bonichon F et al (1997) Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 15:350–362. https://doi.org/10.1200/JCO.1997.15.1.350
Oliveira AM, Nascimento AG (2001) Grading in soft tissue tumors: principles and problems. Skeletal Radiol 30:543–559. https://doi.org/10.1007/s002560100408
Gielen JL, De Schepper AM, Vanhoenacker F et al (2004) Accuracy of MRI in characterization of soft tissue tumors and tumor-like lesions. A prospective study in 548 patients. Eur Radiol 14:2320–2330. https://doi.org/10.1007/s00330-004-2431-0
Berquist TH, Ehman RL, King BF, Hodgman CG, Ilstrup DM (1990) Value of MR imaging in differentiating benign from malignant soft-tissue masses: study of 95 lesions. AJR Am J Roentgenol 155:1251–1255. https://doi.org/10.2214/ajr.155.6.2122675
Vanhoenacker FM, Van Looveren K, Trap K et al (2012) Grading and characterization of soft tissue tumors on magnetic resonance imaging: the value of an expert second opinion report. Insights Imaging 3:131–138. https://doi.org/10.1007/s13244-012-0151-6
Mesko NW, Wilson RJ, Lawrenz JM et al (2018) Pre-operative evaluation prior to soft tissue sarcoma excision—why can’t we get it right? Eur J Surg Oncol 44:243–250. https://doi.org/10.1016/j.ejso.2017.11.001
Dyrop HB, Vedsted P, Rædkjær M, Safwat A, Keller J (2017) Imaging investigations before referral to a sarcoma center delay the final diagnosis of musculoskeletal sarcoma. Acta Orthop 88:211–216. https://doi.org/10.1080/17453674.2016.1278113
Gruber L, Gruber H, Luger AK, Glodny B, Henninger B, Loizides A (2017) Diagnostic hierarchy of radiological features in soft tissue tumours and proposition of a simple diagnostic algorithm to estimate malignant potential of an unknown mass. Eur J Radiol 95:102–110. https://doi.org/10.1016/j.ejrad.2017.07.020
Zhao F, Ahlawat S, Farahani SJ et al (2014) Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology 272:192–201. https://doi.org/10.1148/radiol.14131871
Jahed K, Khazai B, Umpierrez M, Subhawong TK, Singer AD (2018) Pitfalls in soft tissue sarcoma imaging: chronic expanding hematomas. Skeletal Radiol 47:119–124. https://doi.org/10.1007/s00256-017-2770-y
Ahlawat S, Khandheria P, Subhawong TK, Fayad LM (2015) Differentiation of benign and malignant skeletal lesions with quantitative diffusion weighted MRI at 3T. Eur J Radiol 84:1091–1097. https://doi.org/10.1016/j.ejrad.2015.02.019
Singer AD, Pattany PM, Fayad LM, Tresley J, Subhawong TK (2016) Volumetric segmentation of ADC maps and utility of standard deviation as measure of tumor heterogeneity in soft tissue tumors. Clin Imaging 40:386–391. https://doi.org/10.1016/j.clinimag.2015.11.017
Sagiyama K, Watanabe Y, Kamei R et al (2017) Multiparametric voxel-based analyses of standardized uptake values and apparent diffusion coefficients of soft-tissue tumours with a positron emission tomography/magnetic resonance system: preliminary results. Eur Radiol 27:5024–5033. https://doi.org/10.1007/s00330-017-4912-y
Ahlawat S, Khandheria P, Del Grande F et al (2016) Interobserver variability of selective region-of-interest measurement protocols for quantitative diffusion weighted imaging in soft tissue masses: comparison with whole tumor volume measurements. J Magn Reson Imaging 43:446–454. https://doi.org/10.1002/jmri.24994
Fisher S, Wadhwa V, Manthuruthil C, Cheng J, Chhabra A (2016) Clinical impact of magnetic resonance neurography in patients with brachial plexus neuropathies. Br J Radiol 89:20160503. https://doi.org/10.1259/bjr.20160503
Barbieri S, Brönnimann M, Boxler S, Vermathen P, Thoeny HC (2017) Differentiation of prostate cancer lesions with high and with low Gleason score by diffusion-weighted MRI. Eur Radiol 27:1547–1555. https://doi.org/10.1007/s00330-016-4449-5
Li X, Zhang K, Shi Y, Wang F, Meng X (2016) Correlations between the minimum and mean apparent diffusion coefficient values of hepatocellular carcinoma and tumor grade. J Magn Reson Imaging 44:1442–1447. https://doi.org/10.1002/jmri.25323
Kang Y, Choi SH, Kim YJ et al (2011) Gliomas: histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging—correlation with tumor grade. Radiology 261:882–890. https://doi.org/10.1148/radiol.11110686
Teixeira PA, Gay F, Chen B et al (2016) Diffusion-weighted magnetic resonance imaging for the initial characterization of non-fatty soft tissue tumors: correlation between T2 signal intensity and ADC values. Skeletal Radiol 45:263–271. https://doi.org/10.1007/s00256-015-2302-6
Pekcevik Y, Kahya MO, Kaya A (2015) Characterization of soft tissue tumors by diffusion-weighted imaging. Iran J Radiol 12:e15478. https://doi.org/10.5812/iranjradiol.15478v2
van Rijswijk CS, Kunz P, Hogendoorn PC, Taminiau AH, Doornbos J, Bloem JL (2002) Diffusion-weighted MRI in the characterization of soft-tissue tumors. J Magn Reson Imaging 15:302–307
Degnan AJ, Chung CY, Shah AJ (2018) Quantitative diffusion-weighted magnetic resonance imaging assessment of chemotherapy treatment response of pediatric osteosarcoma and Ewing sarcoma malignant bone tumors. Clin Imaging 47:9–13. https://doi.org/10.1016/j.clinimag.2017.08.003
Oka K, Yakushiji T, Sato H, Hirai T, Yamashita Y, Mizuta H (2010) The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol 39:141–146. https://doi.org/10.1007/s00256-009-0830-7
Maeda M, Matsumine A, Kato H et al (2007) Soft-tissue tumors evaluated by line-scan diffusion-weighted imaging: influence of myxoid matrix on the apparent diffusion coefficient. J Magn Reson Imaging 25:1199–1204. https://doi.org/10.1002/jmri.20931
Subhawong TK, Durand DJ, Thawait GK, Jacobs MA, Fayad LM (2013) Characterization of soft tissue masses: can quantitative diffusion weighted imaging reliably distinguish cysts from solid masses? Skeletal Radiol 42:1583–1592. https://doi.org/10.1007/s00256-013-1703-7
Del Grande F, Ahlawat S, Subhawong T, Fayad LM (2017) Characterization of indeterminate soft tissue masses referred for biopsy: what is the added value of contrast imaging at 3.0 tesla? J Magn Reson Imaging 45:390–400
Corino VDA, Montin E, Messina A et al (2018) Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions. J Magn Reson Imaging 47:829–840. https://doi.org/10.1002/jmri.25791
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Avneesh Chhabra, MD.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: AC: consultant, ICON Medical; royalties: Jaypee, Wolters. OA, CS, ND, HW, AC, RS, and YX: no disclosures.
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (YX) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Cross sectional
• Performed at one institution
Rights and permissions
About this article
Cite this article
Chhabra, A., Ashikyan, O., Slepicka, C. et al. Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur Radiol 29, 4485–4494 (2019). https://doi.org/10.1007/s00330-018-5845-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5845-9