Abstract
Gadolinium-based contrast agents (GBCA), widely used in Magnetic Resonance Imaging (MRI) for almost 30 years, were recently shown to be deposited in the brain and to induce persistent T1 shortening in deep gray matter structures in subjects with normal renal function. The aim of the present study is to summarize the evidence derived from the rapidly growing scientific literature on Gadolinium retention in the brain and in the rest of the body. To this end, the original articles that described imaging and pathology findings in humans and animals exposed to GBCA were reviewed. The main aspects that emerged were the different effects of linear and macrocyclic GBCA on brain MRI appearance, the evidence of Gadolinium tissue retention in multiple organs, and the debated issue of the possible clinical consequences. Although no adverse health effects have been documented so far, updated information about GBCA build-up in the body is necessary for health professionals, also in view of the increasing concern in the general population. To date, our knowledge about the mechanisms of Gadolinium tissue deposition and, above all, its long-term consequences is still largely incomplete. However, while official guidelines are eagerly awaited, some advices may already be given, to help our radiological daily practice.


Similar content being viewed by others
References
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reson Imaging 30(6):1240–1248. doi:10.1002/jmri.21966
Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M (2012) MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 36(5):1060–1071. doi:10.1002/jmri.23725
Kanal E (2016) Gadolinium based contrast agents (GBCA): safety overview after 3 decades of clinical experience. Magn Reson Imaging 34(10):1341–1345. doi:10.1016/j.mri.2016.08.017
Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, Cho SH (2012) Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 264(2):414–422. doi:10.1148/radiol.12112025
Bruder O, Schneider S, Pilz G, van Rossum AC, Schwitter J, Nothnagel D, Lombardi M, Buss S, Wagner A, Petersen S, Greulich S, Jensen C, Nagel E, Sechtem U, Mahrholdt H (2015) 2015 Update on acute adverse reactions to gadolinium based contrast agents in cardiovascular MR. Large multi-national and multi-ethnical population experience with 37788 patients from the EuroCMR registry. J Cardiovasc Magn Reson 17:58. doi:10.1186/s12968-015-0168-3
Aime S, Caravan P (2009) Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 30(6):1259–1267. doi:10.1002/jmri.21969
Rogosnitzky M, Branch S (2016) Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 29(3):365–376. doi:10.1007/s10534-016-9931-7
Port M, Idee JM, Medina C, Robic C, Sabatou M, Corot C (2008) Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 21(4):469–490. doi:10.1007/s10534-008-9135-x
Thomsen HS, Morcos SK, Almen T, Bellin MF, Bertolotto M, Bongartz G, Clement O, Leander P, Heinz-Peer G, Reimer P, Stacul F, van der Molen A, Webb JA (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR contrast medium safety committee guidelines. Eur Radiol 23(2):307–318. doi:10.1007/s00330-012-2597-9
Kanda T, Nakai Y, Oba H, Toyoda K, Kitajima K, Furui S (2016) Gadolinium deposition in the brain. Magn Reson Imaging 34(10):1346–1350. doi:10.1016/j.mri.2016.08.024
Tien RD, Brasch RC, Jackson DE, Dillon WP (1989) Cerebral Erdheim-Chester disease: persistent enhancement with Gd-DTPA on MR images. Radiology 172(3):791–792. doi:10.1148/radiology.172.3.2772189
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3):834–841. doi:10.1148/radiol.13131669
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49(10):685–690. doi:10.1097/RLI.0000000000000072
Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, Zobel BB (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol 50(7):470–472. doi:10.1097/RLI.0000000000000154
Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, Takeshita K, Furui S (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275(3):803–809. doi:10.1148/radiol.14140364
Radbruch A, Haase R, Kieslich PJ, Weberling LD, Kickingereder P, Wick W, Schlemmer HP, Bendszus M (2017) No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology 282(3):699–707. doi:10.1148/radiol.2016162241
Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, Heiland S, Wick W, Schlemmer HP, Bendszus M (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275(3):783–791. doi:10.1148/radiol.2015150337
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, Schlemmer HP, Bendszus M (2015) High-Signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Investig Radiol 50(12):805–810. doi:10.1097/RLI.0000000000000227
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, Schlemmer HP, Bendszus M (2016) Intraindividual analysis of signal intensity changes in the dentate nucleus after consecutive serial applications of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol 51(11):683–690. doi:10.1097/RLI.0000000000000308
Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, Radbruch A (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol 50(11):743–748. doi:10.1097/RLI.0000000000000206
Kanal E, Tweedle MF (2015) Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 275(3):630–634. doi:10.1148/radiol.2015150805
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, Semelka RC (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR Images: evaluation of two linear gadolinium-based contrast agents. Radiology 276(3):836–844. doi:10.1148/radiol.2015150872
Ramalho J, Semelka RC, AlObaidy M, Ramalho M, Nunes RH, Castillo M (2016) Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol 26(11):4080–4088. doi:10.1007/s00330-016-4269-7
Huckle JE, Altun E, Jay M, Semelka RC (2016) Gadolinium deposition in humans: when did we learn that gadolinium was deposited in vivo? Invest Radiol 51(4):236–240. doi:10.1097/RLI.0000000000000228
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S (2016) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1 W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26(3):807–815. doi:10.1007/s00330-015-3879-9
Cao Y, Huang DQ, Shih G, Prince MR (2016) Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206(2):414–419. doi:10.2214/AJR.15.15327
Kromrey ML, Liedtke KR, Ittermann T, Langner S, Kirsch M, Weitschies W, Kuhn JP (2017) Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol 27(2):772–777. doi:10.1007/s00330-016-4418-z
Schlemm L, Chien C, Bellmann-Strobl J, Dorr J, Wuerfel J, Brandt AU, Paul F, Scheel M (2016) Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler. doi:10.1177/1352458516670738
Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H (2016) Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Invest Radiol 51(2):83–89. doi:10.1097/RLI.0000000000000242
Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM (2015) Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol 36(10):1859–1865. doi:10.3174/ajnr.A4378
Bae S, Lee HJ, Han K, Park YW, Choi YS, Ahn SS, Kim J, Lee SK (2017) Gadolinium deposition in the brain: association with various GBCAs using a generalized additive model. Eur Radiol. doi:10.1007/s00330-016-4724-5
Flood TF, Stence NV, Maloney JA, Mirsky DM (2017) Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology 282(1):222–228. doi:10.1148/radiol.2016160356
Hu HH, Pokorney A, Towbin RB, Miller JH (2016) Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol 46(11):1590–1598. doi:10.1007/s00247-016-3646-3
Oner AY, Barutcu B, Aykol S, Tali ET (2016) Intrathecal contrast-enhanced magnetic resonance imaging-related brain signal changes: residual gadolinium deposition? Invest Radiol. doi:10.1097/RLI.0000000000000327
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38(3):331–336. doi:10.1016/j.braindev.2015.08.009
Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR (2017) Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology 282(2):516–525. doi:10.1148/radiol.2016152864
Popescu BF, Robinson CA, Rajput A, Rajput AH, Harder SL, Nichol H (2009) Iron, copper, and zinc distribution of the cerebellum. Cerebellum 8(2):74–79. doi:10.1007/s12311-008-0091-3
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13(10):1045–1060. doi:10.1016/S1474-4422(14)70117-6
Zheng W (2001) Toxicology of choroid plexus: special reference to metal-induced neurotoxicities. Microsc Res Tech 52(1):89–103. doi:10.1002/1097-0029(20010101)52:1<89:AID-JEMT11>3.0.CO;2-2
Rasschaert M, Idee JM, Robert P, Fretellier N, Vives V, Violas X, Ballet S, Corot C (2016) Moderate renal failure accentuates T1 signal enhancement in the deep cerebellar nuclei of gadodiamide-treated rats. Invest Radiol. doi:10.1097/RLI.0000000000000339
Kanda T, Oba H, Toyoda K, Kitajima K, Furui S (2016) Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 34(1):3–9. doi:10.1007/s11604-015-0503-5
Khant ZA, Hirai T, Kadota Y, Masuda R, Yano T, Azuma M, Suzuki Y, Tashiro K (2017) T1 shortening in the cerebral cortex after multiple administrations of gadolinium-based contrast agents. Magn Reson Med Sci 16(1):84–86. doi:10.2463/mrms.mp.2016-0054
Eisele P, Alonso A, Szabo K, Ebert A, Ong M, Schoenberg SO, Gass A (2016) Lack of increased signal intensity in the dentate nucleus after repeated administration of a macrocyclic contrast agent in multiple sclerosis: an observational study. Medicine (Baltimore) 95(39):e4624. doi:10.1097/MD.0000000000004624
Tedeschi E, Cocozza S, Borrelli P, Ugga L, Morra VB, Palma G (2017) Longitudinal assessment of Dentate Nucleus relaxometry during massive exposure to gadobutrol. Magn Reson Med Sci. doi:10.2463/mrms.cr.2016-0137
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275(3):772–782. doi:10.1148/radiol.15150025
Jost G, Frenzel T, Lohrke J, Lenhard DC, Naganawa S, Pietsch H (2016) Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol. doi:10.1007/s00330-016-4654-2
Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M (2016) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37(7):1192–1198. doi:10.3174/ajnr.A4615
Tanaka M, Nakahara K, Kinoshita M (2016) Increased signal intensity in the dentate nucleus of patients with multiple sclerosis in comparison with neuromyelitis optica spectrum disorder after multiple doses of gadolinium contrast. Eur Neurol 75(3–4):195–198. doi:10.1159/000445431
Ramalho J, Ramalho M, AlObaidy M, Semelka RC (2016) Technical aspects of MRI signal change quantification after gadolinium-based contrast agent’s administration. Magn Reson Imaging 34(10):1355–1358. doi:10.1016/j.mri.2016.09.004
Tedeschi E, Palma G, Canna A, Cocozza S, Russo C, Borrelli P, Lanzillo R, Angelini V, Postiglione E, Morra VB, Salvatore M, Brunetti A, Quarantelli M (2016) In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol 26(12):4577–4584. doi:10.1007/s00330-016-4245-2
Palma G, Tedeschi E, Borrelli P, Cocozza S, Russo C, Liu S, Ye Y, Comerci M, Alfano B, Salvatore M, Haacke EM, Mancini M (2015) A novel multiparametric approach to 3d quantitative mri of the brain. PLoS One 10(8):e0134963. doi:10.1371/journal.pone.0134963
Borrelli P, Palma G, Tedeschi E, Cocozza S, Comerci M, Alfano B, Haacke EM, Salvatore M (2015) Improving signal-to-noise ratio in susceptibility weighted imaging: a novel multicomponent non-local approach. PLoS One 10(6):e0126835. doi:10.1371/journal.pone.0126835
Cheng HL, Stikov N, Ghugre NR, Wright GA (2012) Practical medical applications of quantitative MR relaxometry. J Magn Reson Imaging 36(4):805–824. doi:10.1002/jmri.23718
Kuno H, Jara H, Buch K, Qureshi MM, Chapman MN, Sakai O (2017) Global and regional brain assessment with quantitative mr imaging in patients with prior exposure to linear gadolinium-based contrast Agents. Radiology 283:195–204. doi:10.1148/radiol.2016160674
Hinoda T, Fushimi Y, Okada T, Arakawa Y, Liu C, Yamamoto A, Yoshida K, Miyamoto S, Togashi K (2016) Quantitative assessment of gadolinium deposition in dentate nucleus using quantitative susceptibility mapping. J Magn Reson Imaging. doi:10.1002/jmri.25490
Ginat DT, Meyers SP (2012) Intracranial lesions with high signal intensity on T1-weighted MR images: differential diagnosis. Radiographics 32(2):499–516. doi:10.1148/rg.322105761
Chen W, Zhu W, Kovanlikaya I, Kovanlikaya A, Liu T, Wang S, Salustri C, Wang Y (2014) Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping. Radiology 270(2):496–505. doi:10.1148/radiol.13122640
Cocozza S, Russo C, Pontillo G, Ugga L, Macera A, Cervo A, De Liso M, Di Paolo N, Ginocchio MI, Giordano F, Leone G, Rusconi G, Stanzione A, Briganti F, Quarantelli M, Caranci F, D’Amico A, Elefante A, Tedeschi E, Brunetti A (2016) Is advanced neuroimaging for neuroradiologists? A systematic review of the scientific literature of the last decade. Neuroradiology 58(12):1233–1239. doi:10.1007/s00234-016-1761-3
Xia D, Davis RL, Crawford JA, Abraham JL (2010) Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol 51(10):1126–1136. doi:10.3109/02841851.2010.515614
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1):228–232. doi:10.1148/radiol.2015142690
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol 51(7):447–453. doi:10.1097/RLI.0000000000000252
Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idee JM, Ballet S, Corot C (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 50(8):473–480. doi:10.1097/RLI.0000000000000181
Robert P, Violas X, Grand S, Lehericy S, Idee JM, Ballet S, Corot C (2016) Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol 51(2):73–82. doi:10.1097/RLI.0000000000000241
Kartamihardja AA, Nakajima T, Kameo S, Koyama H, Tsushima Y (2016) Distribution and clearance of retained gadolinium in the brain: differences between linear and macrocyclic gadolinium based contrast agents in a mouse model. Br J Radiol 89(1066):20160509. doi:10.1259/bjr.20160509
Smith AP, Marino M, Roberts J, Crowder JM, Castle J, Lowery L, Morton C, Hibberd MG, Evans PM (2016) Clearance of gadolinium from the brain with no pathologic effect after repeated administration of gadodiamide in healthy rats: an analytical and histologic study. Radiology. doi:10.1148/radiol.2016160905
Tweedle MF, Wedeking P, Kumar K (1995) Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol 30(6):372–380
Hirano S, Suzuki KT (1996) Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect 104(Suppl 1):85–95
Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE (2009) Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 1(6):479–488. doi:10.1039/b905145g
Gibby WA, Gibby KA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investig Radiol 39(3):138–142
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41(3):272–278. doi:10.1097/01.rli.0000186569.32408.95
Abraham JL, Thakral C, Skov L, Rossen K, Marckmann P (2008) Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol 158(2):273–280. doi:10.1111/j.1365-2133.2007.08335.x
Birka M, Wentker KS, Lusmoller E, Arheilger B, Wehe CA, Sperling M, Stadler R, Karst U (2015) Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem 87(6):3321–3328. doi:10.1021/ac504488k
Christensen KN, Lee CU, Hanley MM, Leung N, Moyer TP, Pittelkow MR (2011) Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 64(1):91–96. doi:10.1016/j.jaad.2009.12.044
Grobner T (2006) Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21(4):1104–1108. doi:10.1093/ndt/gfk062
Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17(9):2359–2362. doi:10.1681/ASN.2006060601
Sanyal S, Marckmann P, Scherer S, Abraham JL (2011) Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review. Nephrol Dial Transplant 26(11):3616–3626. doi:10.1093/ndt/gfr085
Roberts DR, Lindhorst SM, Welsh CT, Maravilla KR, Herring MN, Braun KA, Thiers BH, Davis WC (2016) High levels of gadolinium deposition in the skin of a patient with normal renal function. Invest Radiol 51(5):280–289. doi:10.1097/RLI.0000000000000266
Boyd AS, Sanyal S, Abraham JL (2008) Gadolinium is not deposited in the skin of patients with normal renal function after exposure to gadolinium-based contrast agents. J Am Acad Dermatol 59(2):356–358. doi:10.1016/j.jaad.2008.01.025
Bussi S, Fouillet X, Morisetti A (2007) Toxicological assessment of gadolinium release from contrast media. Exp Toxicol Pathol 58(5):323–330. doi:10.1016/j.etp.2006.09.003
Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, Walter J, Weinmann HJ, Pietsch H (2008) Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 18(10):2164–2173. doi:10.1007/s00330-008-0977-y
Wang YX, Schroeder J, Siegmund H, Idee JM, Fretellier N, Jestin-Mayer G, Factor C, Deng M, Kang W, Morcos SK (2015) Total gadolinium tissue deposition and skin structural findings following the administration of structurally different gadolinium chelates in healthy and ovariectomized female rats. Quant Imaging Med Surg 5(4):534–545. doi:10.3978/j.issn.2223-4292.2015.05.03
Semelka RC, Commander CW, Jay M, Burke LM, Ramalho M (2016) Presumed gadolinium toxicity in subjects with normal renal function: a report of 4 cases. Invest Radiol 51(10):661–665. doi:10.1097/RLI.0000000000000318
Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M (2016) Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn Reson Imaging 34(10):1383–1390. doi:10.1016/j.mri.2016.07.016
Semelka RC, Ramalho M, AlObaidy M, Ramalho J (2016) Gadolinium in humans: a family of disorders. AJR Am J Roentgenol 207(2):229–233. doi:10.2214/AJR.15.15842
Burke LM, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC (2016) Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Reson Imaging 34(8):1078–1080. doi:10.1016/j.mri.2016.05.005
Gathings RM, Reddy R, Santa Cruz D, Brodell RT (2015) Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol 151(3):316–319. doi:10.1001/jamadermatol.2014.2660
Williams SGU (2014) Gadolinium toxicity: a survey of the chronic effects of retained gadolinium from contrast MRIs. https://gdtoxicity.files.wordpress.com/2014/09/gd-symptom-survey.pdf. Accessed 31 May 2016
Gadolinium Toxicity website. www.gadoliniumtoxicity.com. Accessed 10 Jan 2017
Arsenault TM, King BF, Marsh JW Jr, Goodman JA, Weaver AL, Wood CP, Ehman RL (1996) Systemic gadolinium toxicity in patients with renal insufficiency and renal failure: retrospective analysis of an initial experience. Mayo Clin Proc 71(12):1150–1154. doi:10.1016/S0025-6196(11)64695-8
Hui FK, Mullins M (2009) Persistence of gadolinium contrast enhancement in CSF: a possible harbinger of gadolinium neurotoxicity? AJNR Am J Neuroradiol 30(1):E1. doi:10.3174/ajnr.A1205
Maramattom BV, Manno EM, Wijdicks EF, Lindell EP (2005) Gadolinium encephalopathy in a patient with renal failure. Neurology 64(7):1276–1278. doi:10.1212/01.WNL.0000156805.45547.6E
Miller JH, Hu HH, Pokorney A, Cornejo P, Towbin R (2015) MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics 136(6):e1637–1640. doi:10.1542/peds.2015-2222
Balint B, Bhatia KP (2016) T1-weighted basal ganglia hyperintensities due to gadolinium deposition—a cautionary note. Parkinsonism Relat Disord 32:135–136. doi:10.1016/j.parkreldis.2016.09.017
Welk B, McArthur E, Morrow SA, MacDonald P, Hayward J, Leung A, Lum A (2016) Association between gadolinium contrast exposure and the risk of parkinsonism. JAMA 316(1):96–98. doi:10.1001/jama.2016.8096
US Food and Drug Administration FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). www.fda.gov/downloads/Drugs/DrugSafety/UCM455390.pdf. Accessed 28 June 2016
Malayeri AA, Brooks KM, Bryant LH, Evers R, Kumar P, Reich DS, Bluemke DA (2016) National Institutes of health perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol 13(3):237–241. doi:10.1016/j.jacr.2015.11.009
AuntMinnieEurope.com EMA continues investigation of gadolinium contrast agents. http://www.auntminnieeurope.com/index.aspx?sec=ser&sub=def&pag=dis&ItemID=613908. Accessed 18 Jan 2017
Quattrocchi CC, van der Molen AJ (2017) Gadolinium retention in the body and brain: is It time for an international joint research effort? Radiology 282(1):12–16. doi:10.1148/radiol.2016161626
Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, Squillaci E, Briganti F, Brunese L (2015) Errors in neuroradiology. Radiol Med 120(9):795–801. doi:10.1007/s11547-015-0564-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no funding.
Conflict of interest
All authors (Enrico Tedeschi, Ferdinando Caranci, Flavio Giordano, Valentina Angelini, Sirio Cocozza and Arturo Brunetti) declare that they have no conflict of interest.
Informed consent
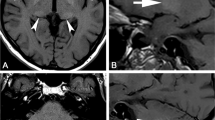
The MRI scans shown in the figures were retrospectively selected among MRI studies previously performed according to clinical indications, with informed consent obtained from all individual participants.
Ethical statements
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tedeschi, E., Caranci, F., Giordano, F. et al. Gadolinium retention in the body: what we know and what we can do. Radiol med 122, 589–600 (2017). https://doi.org/10.1007/s11547-017-0757-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0757-3