Abstract
Objectives
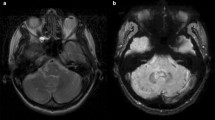
To evaluate changes in T1 and T2* relaxometry of dentate nuclei (DN) with respect to the number of previous administrations of Gadolinium-based contrast agents (GBCA).
Methods
In 74 relapsing-remitting multiple sclerosis (RR-MS) patients with variable disease duration (9.8±6.8 years) and severity (Expanded Disability Status Scale scores:3.1±0.9), the DN R1 (1/T1) and R2* (1/T2*) relaxation rates were measured using two unenhanced 3D Dual-Echo spoiled Gradient-Echo sequences with different flip angles. Correlations of the number of previous GBCA administrations with DN R1 and R2* relaxation rates were tested, including gender and age effect, in a multivariate regression analysis.
Results
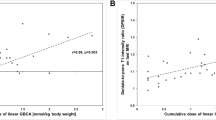
The DN R1 (normalized by brainstem) significantly correlated with the number of GBCA administrations (p<0.001), maintaining the same significance even when including MS-related factors. Instead, the DN R2* values correlated only with age (p=0.003), and not with GBCA administrations (p=0.67). In a subgroup of 35 patients for whom the administered GBCA subtype was known, the effect of GBCA on DN R1 appeared mainly related to linear GBCA.
Conclusions
In RR-MS patients, the number of previous GBCA administrations correlates with R1 relaxation rates of DN, while R2* values remain unaffected, suggesting that T1-shortening in these patients is related to the amount of Gadolinium given.
Key Points
• In multiple sclerosis, previous Gadolinium administrations correlate with dentate nuclei T1 relaxometry.
• Such correlation is linked to linear Gadolinium chelates and unrelated to disease duration or severity.
• Dentate nuclei T2* relaxometry is age-related and independent of previous Gadolinium administrations.
• Changes in dentate nuclei T1 relaxometry are not determined by iron accumulation.
• MR relaxometry can quantitatively assess Gadolinium accumulation in dentate nuclei.



Similar content being viewed by others
Abbreviations
- BBB:
-
Blood-Brain-Barrier
- CNS:
-
Central Nervous System
- CE-MRI:
-
Contrast-Enhanced MR scans
- DN:
-
Dentate Nuclei
- DD:
-
Disease Duration
- EDSS:
-
Expanded Disability Status Scale
- FLAIR:
-
Fluid Attenuated Inversion Recovery
- GRE:
-
Gradient Echo
- Gd:
-
Gadolinium
- GBCA:
-
Gadolinium-Based Contrast Agents
- GM:
-
Gray Matter
- MPRAGE:
-
Magnetization Prepared Rapid Acquisition Gradient Echo
- MRI:
-
Magnetic Resonance Imaging
- ms:
-
milliseconds
- MS:
-
Multiple Sclerosis
- NSF:
-
Nephrogenic Systemic Fibrosis
- RR-MS:
-
Relapsing-Remitting Multiple Sclerosis
- nR1:
-
Normalized R1 relaxation rate
- SP-MS:
-
Secondary-Progressive Multiple Sclerosis
- WM:
-
White Matter
References
Kanal E, Maravilla K, Rowley HA (2014) Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 35:2215–2226
Giesel FL, Mehndiratta A, Essig M (2010) High-relaxivity contrast-enhanced magnetic resonance neuroimaging: a review. Eur Radiol 20:2461–2474
Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M (2012) MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 36:1060–1071
Brown RW, Cheng Y C N, Haacke E M, Thompson M R, Venkatesan R (2014) Magnetic Resonance Imaging: Physical Principles and Sequence Design. John Wiley & Sons
Reiser MF, Semmler, W, Hricak, H (2008) Magnetic Resonance Tomography. Springer
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49:685–690
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Kanda T, Osawa M, Oba H et al (2015) High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology 275:803–809
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Ramalho J, Castillo M, AlObaidy M et al (2015) High Signal Intensity in Globus Pallidus and Dentate Nucleus on Unenhanced T1-weighted MR Images: Evaluation of Two Linear Gadolinium-based Contrast Agents. Radiology:150872
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S (2015) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol
Weberling LD, Kieslich PJ, Kickingereder P et al (2015) Increased Signal Intensity in the Dentate Nucleus on Unenhanced T1-Weighted Images After Gadobenate Dimeglumine Administration. Invest Radiol
Radbruch A, Weberling LD, Kieslich PJ et al (2015) High-Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted Images: Evaluation of the Macrocyclic Gadolinium-Based Contrast Agent Gadobutrol. Invest Radiol 50:805–810
Tofts P (2005) Quantitative MRI of the brain: measuring changes caused by disease. John Wiley & Sons
Ginat DT, Meyers SP (2012) Intracranial lesions with high signal intensity on T1-weighted MR images: differential diagnosis. Radiographics 32:499–516
Ogg RJ, McDaniel CB, Wallace D, Pitot P, Neel MD, Kaste SC (2005) MR safety and compatibility of a noninvasively expandable total-joint endoprosthesis. Magn Reson Imaging 23:789–794
Diedrichsen J, Maderwald S, Kuper M et al (2011) Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage 54:1786–1794
Maschke M, Weber J, Dimitrova A et al (2004) Age-related changes of the dentate nuclei in normal adults as revealed by 3D fast low angle shot (FLASH) echo sequence magnetic resonance imaging. J Neurol 251:740–746
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060
Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL (2009) Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology 251:503–510
Borrelli P, Palma G, Tedeschi E et al (2015) Improving Signal-to-Noise Ratio in Susceptibility Weighted Imaging: A Novel Multicomponent Non-Local Approach. PLoS ONE 10, e0126835
Palma G, Tedeschi E, Borrelli P et al (2015) A Novel Multiparametric Approach to 3D Quantitative MRI of the Brain. PLoS ONE 10, e0134963
Tjoa CW, Benedict RH, Weinstock-Guttman B, Fabiano AJ, Bakshi R (2005) MRI T2 hypointensity of the dentate nucleus is related to ambulatory impairment in multiple sclerosis. J Neurol Sci 234:17–24
Langkammer C, Krebs N, Goessler W et al (2010) Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257:455–462
Khalil M, Langkammer C, Ropele S et al (2011) Determinants of brain iron in multiple sclerosis: a quantitative 3T MRI study. Neurology 77:1691–1697
Quattrocchi CC, Mallio CA, Errante Y et al (2015) Gadodiamide and Dentate Nucleus T1 Hyperintensity in Patients With Meningioma Evaluated by Multiple Follow-Up Contrast-Enhanced Magnetic Resonance Examinations With No Systemic Interval Therapy. Invest Radiol 50:470–472
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 275:772–782
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 276:228–232
Kanal E, Tweedle MF (2015) Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 275:630–634
Birka M, Wentker KS, Lusmoller E et al (2015) Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem 87:3321–3328
van Horssen J, Brink BP, de Vries HE, van der Valk P, Bo L (2007) The blood-brain barrier in cortical multiple sclerosis lesions. J Neuropathol Exp Neurol 66:321–328
Cheng HL, Stikov N, Ghugre NR, Wright GA (2012) Practical medical applications of quantitative MR relaxometry. J Magn Reson Imaging 36:805–824
Deoni SC (2010) Quantitative relaxometry of the brain. Top Magn Reson Imaging 21:101–113
Bonnier G, Roche A, Romascano D et al (2014) Advanced MRI unravels the nature of tissue alterations in early multiple sclerosis. Ann Clin Transl Neurol 1:423–432
Walsh AJ, Blevins G, Lebel RM, Seres P, Emery DJ, Wilman AH (2014) Longitudinal MR imaging of iron in multiple sclerosis: an imaging marker of disease. Radiology 270:186–196
Deoni SC, Mercure E, Blasi A et al (2011) Mapping infant brain myelination with magnetic resonance imaging. J Neurosci 31:784–791
Saito N, Sakai O, Ozonoff A, Jara H (2009) Relaxo-volumetric multispectral quantitative magnetic resonance imaging of the brain over the human lifespan: global and regional aging patterns. Magn Reson Imaging 27:895–906
Davies GR, Hadjiprocopis A, Altmann DR et al (2007) Normal-appearing grey and white matter T1 abnormality in early relapsing-remitting multiple sclerosis: a longitudinal study. Mult Scler 13:169–177
Manfredonia F, Ciccarelli O, Khaleeli Z et al (2007) Normal-appearing brain t1 relaxation time predicts disability in early primary progressive multiple sclerosis. Arch Neurol 64:411–415
Vrenken H, Geurts JJ, Knol DL et al (2006) Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology 240:811–820
Absinta M, Rocca MA, Filippi M (2011) Dentate nucleus T1 hyperintensity in multiple sclerosis. AJNR Am J Neuroradiol 32:E120–121
Yan Y, Shao E, Deng X, Liu J, Zhang Y, Tang Y (2014) Microwave-assisted synthesis of Gd(III)-loaded nanozeolite SOD as MRI contrast agent with remarkable stability in vivo. J Mater Chem B 2:3041–3049
Arsenault TM, King BF, Marsh JW Jr et al (1996) Systemic gadolinium toxicity in patients with renal insufficiency and renal failure: retrospective analysis of an initial experience. Mayo Clin Proc 71:1150–1154
Maramattom BV, Manno EM, Wijdicks EF, Lindell EP (2005) Gadolinium encephalopathy in a patient with renal failure. Neurology 64:1276–1278
Swaminathan S, High WA, Ranville J et al (2008) Cardiac and vascular metal deposition with high mortality in nephrogenic systemic fibrosis. Kidney Int 73:1413–1418
Ropele S, Kilsdonk ID, Wattjes MP et al (2014) Determinants of iron accumulation in deep grey matter of multiple sclerosis patients. Mult Scler 20:1692–1698
Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R (2010) MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49:1271–1281
Acknowledgments
The help of Raffaele Palladino, MD in reviewing the statistical analyses is gratefully acknowledged. The scientific guarantor of this publication is Enrico Tedeschi, MD. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by Italian Ministry of Education, University, and Research (MIUR) within the PRIN framework (2010XE5L2R). One of the authors (MQ) has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tedeschi, E., Palma, G., Canna, A. et al. In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol 26, 4577–4584 (2016). https://doi.org/10.1007/s00330-016-4245-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4245-2