Abstract
Purpose of Review
Gadolinium-based contrast agents (GBCAs) have been used since 1988 for magnetic resonance imaging. These agents were considered very safe and, excluding nephrogenic systemic fibrosis in patients with renal failure, no clinical entities were associated with its administration. Our purpose is to summarize gadolinium induced toxicity by reviewing the most recent literature in this subject.
Recent Findings
It was believed that GBCAs distribute rapidly in the extracellular volume after intravenous administration and eliminate rapidly, almost completely, via renal excretion. Currently, we know that this is not entirely true. Some GBCAs may be retained, undergo dechelation and induce gadolinium deposition in different tissues such as liver, bone or brain in patients with normal renal function. The retained gadolinium, whether dechelated and associated with different compounds or in its original formulation, may trigger gadolinium-related toxic effects. This potential toxicity became a global concern.
Summary
This review summarizes the most recent published data regarding gadolinium deposition, possible clinical significance of gadolinium tissue retention and accumulation, strategies to limit gadolinium body burden and some future trends in this area.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Zobel BB, Quattrocchi CC, Errante Y, Grasso RF. Gadolinium-based contrast agents: did we miss something in the last 25 years? Radiol Med. Springer Milan; 2016Feb.4:1–4. This is a brief review of GBCAs in daily practice.
Runge VM. C Commentary on T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol. 2015;50(8):481–2.
Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol. 2004;39(3):138–42.
White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41(3):272–8.
Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009;1(6):479.
Rocklage SM, Worah D, Kim SH. Metal ion release from paramagnetic chelates: what is tolerable? Magn Reson Med. 1991;22(2):216–32.
•• Tweedle MF, Kanal E and Muller R. Considerations in the selection of a new gadolinium-based contrast agent. Appl Radiol 2014May:1-11 This is a comprehensive overview regarding GBCAs selection.
Frenzel T, Lengsfeld P, Schirmer H, Hütter J. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 C. Invest Radiol. 2008;43:817–28.
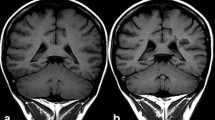
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced t1-weighted mr images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–41.
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014;49(10):685–90.
Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, et al. Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol. 2015;50(7):470–2.
Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology. 2015;275(3):803–9.
Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015;275(3):783–91.
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1 W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol. 2016;26(3):807–15.
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images. Invest Radiol. 2015;50(12):805–10.
Cao Y, Huang DQ, Shih G, Prince MR. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. Am J Roentgenol. 2016;206(2):414–9.
Kromrey M-L, Liedtke KR, Ittermann T, Langner S, Kirsch M, Weitschies W, et al. Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol. European Radiology; 2016;1–6.
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, et al. High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology. 2015;276(3):836–44.
Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer H-P, et al. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol. 2015;50(11):743–8.
Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM. Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol. 2015;36(10):1859–65.
Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain and Development. Jpn Soc Child Neurol. 2015;38(3):331–6.
Miller JH, Hu HH, Pokorney A, Cornejo P, Towbin R. MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics. 2015;136(6):e1637–40.
Hu HH, Pokorney A, Towbin RB, Miller JH. Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol. 2016;8:1–9.
Jessome R. Hyperintensity in the dentate nucleus on nonenhanced T1-weighted magnetic resonance imaging suggests dechelation of contrast agents. J Med Imaging Radiat Sci. 2016;5:1–6.
Ramalho J, Semelka RC, AlObaidy M, Ramalho M, Nunes RH, Castillo M. Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol. 2016..
• McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015;275(3):772–82. This was the first human histopathological study confirming gadolinium deposition in patients with normal renal function.
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276(1):228–32.
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue. Invest Radiol. 2016;51(7):447–53.
Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idée J-M, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol. 2015;50(8):473–80.
• Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in Humans: A Family of Disorders. American Journal of Roentgenology. 2016 (on line). In this paper the authors propose a nomenclature for gadolinium-induced entities.
• Burke LMB, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC. Self-reported gadolinium toxicity: A survey of patients with chronic symptoms. Magn Reson Imaging. Elsevier Inc; 2016;34(8):1078–80. This is the first report of clinical symptoms eventually related with gadolinium toxicity.
Altun E, Martin DR, Wertman R, Lugo-Somolinos A, Fuller ER, Semelka RC, et al. Nephrogenic systemic fibrosis: change in incidence following a switch in gadolinium agents and adoption of a gadolinium policy–report from two U.S. universities. Invest Radiol. 2015;253(3):689–96.
Kanal E, Tweedle MF. Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology. 2015;275(3):630–4.
•• Runge VM. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Invest Radiol. 2016;51(5):273–9 This is a comprehensive overview of GBCAs stability and gadolinium deposition in brain.
Reeder SB, Gulani V. Gadolinium deposition in the brain: do we know enough to change practice? Radiology. 2016;279(1):323–6.
Kanda T, Oba H, Toyoda K, Kitajima K, Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol. 2016;34(1):1–7.
• Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. Springer Netherlands; 2016;29(3):365–76 This is an important overview that examines the potential biochemical and molecular basis of gadolinium toxicity and possible clinical significance of gadolinium tissue retention and accumulation.
Port M, Idée J-M, Medina C, Robic C, Sabatou M, Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008;21(4):469–90.
Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18(10):2164–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joana Ramalho and Miguel Ramalho declare no potential conflicts of interest. Richard C. Semelka reports research support from Siemens and personal fees from Guerbet and Bracco. Richard C. Semelka is a section editor for Current Radiology Reports.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Global Radiology Concerns: Income, Academics, Quality, Safety.
Rights and permissions
About this article
Cite this article
Ramalho, J., Ramalho, M. & Semelka, R.C. Gadolinium Deposition and Toxicity: A Global Concern. Curr Radiol Rep 4, 59 (2016). https://doi.org/10.1007/s40134-016-0187-3
Published:
DOI: https://doi.org/10.1007/s40134-016-0187-3