Abstract
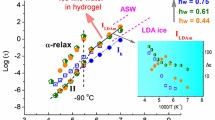
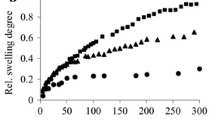
Glass transition and water dynamics in hydrated hyaluronic acid (HA) hydrogels crosslinked by divinyl sulfone (DVS) were studied by differential scanning calorimetry (DSC), dielectric relaxation spectroscopy (DRS) and water sorption—desorption (ESI) measurements. A critical water fraction of about h w = 0.17 (g of water per g of hydrated HA) for a change in the hydration properties of the material was estimated. Water crystallization was recorded by DSC during cooling and heating for water fraction values h w ≥ 0.31. The glass transition of the hydrated system was recorded in the water fraction region 0.06 ≤ h w ≤ 0.59. The T g was found to decrease with increasing hydration level, starting from T g = −48 °C down to about T g = −80 °C and then to stabilize there, for the hydration levels where water crystallization occurs, suggesting that the origin of the glass transition is the combined motion of uncrystallized water molecules attached to primary hydration sites and segments of the HA chains. DRS studies revealed two relaxation peaks, associated with the main secondary relaxation process of uncrystallized water molecules (UCW) triggering the mobility of polar groups and the segmental mobility of HA chains (α relaxation). The α relaxation was in good agreement with the results by DSC. A qualitative change in the dynamics of the α relaxation was found for h w = 0.23 and was attributed to a reorganization of water in the material due to structural changes. Finally, the dielectric strength of the relaxation of UCW was found to decrease in the water fraction region of the structural changes, i.e. for h w ~ 0.23.












Similar content being viewed by others
References
T.C. Laurent, Ciba Foundation Symposium, vol. 143 (John Wiley and Sons, New York, 1989), pp. 1–298
J. Necas, L. Bartosikov, P. Brauner, J. Kolar, Vet. Med. 53(8), 397–411 (2008)
M.K. Cowman, M. Li, E.A. Balazs, Biophys. J. 75, 2030–2037 (1998)
M.K. Cowman, S. Matsuoka, Carbohydr. Res. 340, 791–809 (2005)
C.E. Schanté, G. Zuber, C. Herlin, T.F. Vendamme, Carbohydr. Polym. 85, 469–489 (2011)
E.J. Oh, K. Park, K.S. Kim, J. Kim, J.-A. Yang, J.-H. Kong, M.Y. Lee, A.S. Hoffman, S.K. Hahn, J. Control. Release 141, 2–12 (2010)
A.S. Hoffman, Adv. Drug Deliv. Rev. 54, 3–12 (2002)
F. Lee, M. Kurisawa, Acta Biomaterialia 9(2), 5143–5152 (2013)
H.N. Joshi, E.M. Topp, Int. J. Pharm. 80, 213–225 (1992)
J. Kucerik, A. Prusova, A. Rotaru, K. Flimel, J. Janecek, P. Conte, Thermochim. Acta 523, 245–249 (2011)
M.N. Collins, C. Birkinshaw, J. Mater. Sci. Mater. Med. 19, 3335–3343 (2008)
R. Servaty, J. Schiller, H. Binder, K. Arnold, Int. J. Biol. Macromol. 28, 121–127 (2001)
J. Kaufmann, K. Möhle, H.J. Hofmann, K. Arnold, J. Mol. Struct. (THEOCHEM) 422, 109–121 (1998)
H. Sugimoto, T. Miki, K. Κanayama, M. Norimoto, J. Non-Cryst. Solids 354, 3220–3224 (2008)
J. Mijović, Y. Bian, R.A. Gross, B. Chen, Macromolecules 38, 10812–10819 (2005)
J. Swenson, H. Jansson, J. Hedström, R. Bergman, J. Phys. Condens. Matter 19, 205109–205117 (2007)
C. Gainaru, A. Fillmer, R. Böhmer, J. Phys. Chem. B 113, 12628–12631 (2009)
W. Doster, S. Busch, A.M. Gaspar, M.S. Appavu, J. Wuttke, H. Scheer, Phys. Rev. Lett. 104, 098101–098104 (2010)
A. Panagopoulou, A. Kyritsis, N. Shinyashiki, P. Pissis, J. Phys. Chem. B 116, 4593–4602 (2012)
P. Pissis, A. Kyritsis, J. Polym. Sci. B Polym. Phys. 51(3), 159–175 (2013)
G. Careri, Prog. Biophys. Mol. Biol. 70, 223–249 (1998)
S. Cerveny, A. Alegria, J. Colmenero, Phys. Rev. E 77, 031803–031807 (2008)
K.L. Ngai, S. Capaccioli, S. Ancherbak, N. Shinyashiki, Phil. Mag. 91, 1809–1835 (2011)
A. Panagopoulou, A. Kyritsis, A.M. Aravantinou, D. Nanopoulos, R. Sabater i Serra, J.L. Gómez Ribellez, N. Shinyashiki, P. Pissis, Food Biophys. 6, 199–209 (2011)
A. Panagopoulou, A. Kyritsis, R. Sabater i Serra, J.L. Gómez Ribellez, N. Shinyashiki, P. Pissis, Biochim. Biophys. Acta 1814, 1984–1996 (2011)
R.B. Gregory, Protein-Solvent Interactions (Marcel Dekker, New York, USA, 1995)
D. Ringe, G.A. Petsko, Biophys. Chem. 105, 667–680 (2003)
P.W. Fenimore, H. Frauenfelder, B.H. McMahon, R.D. Young, Proc. Natl. Acad. Sci. 101, 14408–14413 (2004)
Y. Miyazaki, T. Matsuo, H. Suga, J. Phys. Chem. B 104, 8044–8052 (2000)
N. Shinyashiki, W. Yamamoto, A. Yokoyama, T. Yoshinari, S. Yagihara, K.L. Ngai, S. Capaccioli, J. Phys. Chem. B 113, 14448–14456 (2009)
S. Khodadadi, A. Malkovskiy, A. Kisliuk, A.P. Sokolov, Biochim. Biophys. Acta 1804, 15–19 (2010)
H. Jansson, J. Swenson, Biochim. Biophys. Acta 1804, 20–26 (2010)
A.L. Tournier, J. Xu, J.C. Smith, Biophys. J. 85, 1871–1875 (2003)
D. Porter, F. Vollrath, Biochim. Biophys. Acta 1824, 785–791 (2012)
T. Vuletić, S. Dolanski Babić, T. Ivek, D. Grgičin, S. Tomić, Phys. Rev. E 82, 011922–011932 (2010)
L. Greenspan, Humidity fixed points of binary saturated aqueous solutions. J. Res. Nat. Bur. Stand. A Phys. Chem. 81A, 89–96 (1977)
F. Kremer, A. Schönhals (eds.), Broadband Dielectric Spectroscopy (Springer, Berlin, 2002)
R.H. Cole, K.S. Cole, J. Chem. Phys. 10, 98–105 (1942)
R.P. Chartoff, P.T. Weissman, A. Sirkar, in The Application of Dynamic Mechanical Methods to T g Determination in polymers: An overview, Assignment of the Glass Transition, ASTM STP 1249, ed. by R.J. Seyler (American Society for Testing and Materials, Philadelphia, 1994), pp. 88–107
H. Vogel, Phys. Z. 22, 645–646 (1921)
A. Anagnostopoulou-Konsta, P. Pissis, J. Phys. D. Appl. Phys. 20, 1168–1174 (1987)
D. Daoukaki-Diamanti, P. Pissis, G. Boudouris, Chem. Phys. 91, 315–325 (1984)
P. Pissis, J. Phys. D. Appl. Phys. 18, 1897–1908 (1985)
S. Ratkovic, P. Pissis, J. Mater. Sci. 32, 3061–3068 (1997)
P. Pissis, J. Exp. Bot 41, 677–684 (1990)
Acknowledgments
This research has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund (A.P. and P.P.) and Research Funding Program: Aristeia (A.K. and P.P.). A.V.L. and M.M.P. acknowledge financial support of projects MAT2011-28791-C03-02 and -03.
The authors would like to acknowledge Sara Poveda Reyes and María Hernández Palacios for the preparation of the HA sheets.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panagopoulou, A., Molina, J.V., Kyritsis, A. et al. Glass Transition and Water Dynamics in Hyaluronic Acid Hydrogels. Food Biophysics 8, 192–202 (2013). https://doi.org/10.1007/s11483-013-9295-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-013-9295-2