Abstract
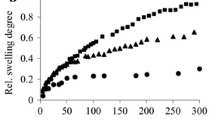
In order to improve the mechanical properties and control the degradation rate of hyaluronic acid (HA) an investigation of the structural and mechanical properties of the hydrogels crosslinked using divinyl sulfone (DVS), glutaraldehyde (GTA) and freeze-thawing, or autocrosslinking has been carried out. The thermal and mechanical properties of the gels were characterised by differential scanning calorimetry (DSC), dynamic mechanical thermal analysis (DMTA) and compression tests. The solution degradation products of each system have been analysed using size exclusion chromatography (SEC) and the Zimm–Stockmayer theory applied. Autocrosslinked gels swell the most quickly, whereas the GTA crosslinked gels swell most slowly. The stability of the autocrosslinked gels improves with a reduction in solution pH, but is still poor. GTA and DVS crosslinked gels are robust and elastic when water swollen, with glass transition values around 20°C. SEC results show that the water soluble degradation products of the gels show a reduction in the radius of gyration at any particular molecular weight and this is interpreted as indicating increased hydrophobicity arising from chemical modification.














Similar content being viewed by others
References
A. Lowman, N. Peppas, Hydrogels, in Encyclopedia of Controlled Drug Delivery, ed. by E. Mathiowitz (New York, Wiley, 1999), pp. 397–418
J.H. deGoot et al., Meniscal tissue regeneration in porous 50/50 copoly(l-lactide/caprolactone) implants. Biomaterials 18(8):613–622 (1997)
D. Ingber et al., Chapter 2. in Physical Forces and the Mammalian Cell. (Academic Press, New York, 1993)
L. Lapcik, et al., Hyaluronan: preparation, styructure, properties and applications. Chem. Rev. 98(8), 2663–2684 (1998)
W.Y. Chen, G. Abatangelo, Functions of hyaluronan in wound repair. Wound Repair Regen. 7, 79–89 (1999)
E.A. Turley, P.W. Nobel, Signalling properties of hyaluronan receptors. J. Biol. Chem. 277, 4589–4592 (2002)
F.S. Palumbo, et al., New graft copolymers of hyaluronic acid and polylactic acid: synthesis and characterization. Carbohydr. Polym. 66(3), 379–385 (2006)
S.-N. Park et al., Biological characterization of EDC-crosslinked collagen–hyaluronic acid matrix in dermal tissue restoration. Biomaterials 24(9), 1631–1641 (2003)
J. Leach, et al., Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 82(5), 578–589 (2003)
T.C. Laurent (ed.), The Chemistry, Biology and Medical Applcations of Hyaluronan and its Derivatives. Wenner-Gren International Series, vol. 72 (Portland Press Ltd, London, 1998)
J.C. Fernandez Lopez, A. Ruano-Ravina, Efficacy and safety of intraarticular hyaluronic acid in the treatment of hip osteoarthritis: a systematic review. Osteoarth. Cart. 14(12), 1306–1311 (2006)
R. Barbucci et al., Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials 23(23), 4503–4513 (2002)
E. Balazs, in Sodium Hyaluronate and Viscosurgery. Meds. Healon (Sodium Hyaluronate). A Guide to Its Use in Ophthalmic Surgery, ed. by D. Miller, R. Stegmann (Wiley, New York, 1983), pp. 5–28
E. Turley, RHAMM, a Member of the Hyaladherins. in The Science of Hyaluronan Today. 1999 http://www.glycoforum.gr.jp/index.htm
L. Huang, et al., Engineered collagen-PEO nanofibrils and fabrics. J. Biomat. Sci.: Polym. Ed. 12, 979–993 (2001)
O. Oksala, et al., Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 43(2), 125–135 (1995)
K. Fukuda, et al., Hyaluronic acid inhibits interleukin-1-induced superoxide anion in bovine chondrocytes. Inflamm. Res. 46(3), 114–117 (1997)
K. Tomihata, Y. Ikada, Crosslinking of hyaluronic acid with glutaraldehyde. J. Polym. Sci. A: Polym. Chem. 35, 3553–3559 (1997)
K. Tomihata, Y. Ikada, Preparation of crosslinked hyaluronic acid films of low water content. Biomaterials 18(3), 189–195 (1997)
K. Tomihata, Y. Ikada, Crosslinking of hyaluronic acid with water soluble carbodiimide. J. Biomed. Mater. Res. 37, 243–251 (1997)
M.N. Collins, C. Birkinshaw, Comparison of the effectiveness of four different crosslinking agents with hyaluronic acid hydrogel films for tissue-culture applications. J. Appl. Polym. Sci. 104, 3183–3191 (2007)
M.N. Collins, C. Birkinshaw, Investigation of the swelling behaviour of crosslinked hyaluronic acid films and hydro-gels produced using homogeneous reactions. J. Appl. Polym. Sci. 109, 923–931 (2008)
A. Okamoto, T. Miyoshi, A Biocompatible gel of Hyaluronan. in Hyaluronan, ed. by J. Kennedy, G. Phillips, P. Williams (Woodhead Publishing Limited, Cambridge, 2002)
J. Bracke, K. Thacker, Hyaluronic Acid from Bacterial Culture (Diagnostic Inc, Minneapolis, MN, USA, 1985)
J.W. Burns, S. Cox, A. Walts, In United States Patent 5,017,229 (Genzyme Corporation, Cambridge, MA, USA, 1991)
J. Roovers, Branched Polymers, in Encylopedia of Polymer Science & Eng., vol. 2 (John Wiley, 1985), pp. 478–499
S. Grcev, P. Schoenmakers, P. Iedema, Determination of molecular weight and size distribution and branching characteristics of PVAc by means of size exclusion chromatography/multi-angle laser light scattering (SEC/MALLS). Polymer 45, 39–48 (2004)
M.N. Collins, in Fabrication of Porous Scaffolds for Tissue Engineering. PhD thesis (University of Limerick, 2007)
S.A. Bencherif, A. Srinivasan, F. Horkay, J.O. Hollinger, K. Matyjaszewski, N.R. Washburn, Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 29, 1739–1749 (2008)
K.P.Menard, Dynamic Mechanical Analysis Basics: Part 2 Thermoplastic Transitions and Properties. Perkin Elmer: Application Note (1999)
K. Kirker, G. Prestwich, Physical properties of glycosaminoglycan hydrogels. J. Polym. Sci. B: Polym. Phys. 42, 4344–4356 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Collins, M.N., Birkinshaw, C. Physical properties of crosslinked hyaluronic acid hydrogels. J Mater Sci: Mater Med 19, 3335–3343 (2008). https://doi.org/10.1007/s10856-008-3476-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3476-4