Abstract
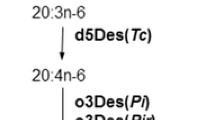
The synthesis of GLA (Δ6, 9, 12-18:3) is carried out in a number of plant taxa by introducing a double bond at the Δ6 position of its precursor, linoleic acid (Δ9, 12-18:2), through a reaction catalyzed by a Δ6-desaturase enzyme. We have cloned genes encoding the Δ6-desaturase (D6DES) from two different Macaronesian Echium species, E. pitardii and E. gentianoides (Boraginaceae), which are characterized by the accumulation of high amounts of GLA in their seeds. The Echium D6DES genes encode proteins of 438 amino acids bearing the prototypical cytochrome b5 domain at the N-terminus. Cladistic analysis of desaturases from higher plants groups the Echium D6DES proteins together with other Δ6-desaturases in a different cluster from that of the highly related Δ8-desaturases. Expression analysis carried out in E. pitardii shows a positive correlation between the D6DES transcript level and GLA accumulation in different tissues of the plant. Although a ubiquitous expression in all organs is observed, the transcript is particularly abundant in developing fruits, whereas a much lower level is present in mature leaves. Functional characterization of the D6DES gene from E. gentianoides has been achieved by heterologous expression in tobacco plants and in the yeast Saccharomyces cerevisiae. In both cases, overexpression of the gene led to the synthesis of GLA. Biotechnological application of these results can be envisaged as an initial step toward the generation of transgenic oleaginous plants producing GLA.
Similar content being viewed by others
Abbreviations
- ALA:
-
α-linolenic acid
- CaMV:
-
cauliflower mosaic virus
- DIG:
-
digoxigenin
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- IPCR:
-
inverse polymerase chain reaction
- LA:
-
Imoleic acid
- MS:
-
mass spectrometry
- OTA:
-
octadecatetraenoic acid
- PCR:
-
polymerase chain reaction
References
Gunstone, F.D. (1992) γ-Linolenic Acid-Occurrence and Physical and Chemical Properties, Prog. Lipid Res. 31, 145–161.
Gunstone, F.D. (1998) Movements Towards Tailor-Made Fats, Prog. Lipid Res. 37, 277–305.
Horrobin, D.F. (1992) Nutritional and Medical Importance of γ-Linolenic Acid, Prog. Lipid Res. 31, 163–194.
Brosche, T., and Platt, D. (2000) Effect of Borage Oil Consumption on Fatty Acid Metabolism, Transepidermal Water Loss and Skin Parameters in Elderly People, Arch. Gerontol. Geriatr. 30, 139–150.
Takahashi, Y., Ide, T., and Fujita, H. (2000) Dietary γ-Linolenic Acid in the Form of Borage Oil Causes Less Body Fat Accumulation Accompanying an Increase in Uncoupling Protein 1 mRNA Level in Brown Adipose Tissue, Comp. Biochem. Physiol. 127, 213–222.
Das, U.N., Prasad, V.V.S.K., and Reddy, D.R. (1995) Local Application of γ-Linolenic Acid in the Treatment of Human Gliomas, Cancer Lett. 94, 147–155.
Preuss, M., Girnun, G.D., Darby, C.J., Khoo, N., Spector, A.A., and Robbins, M.E. (2000) Role of Antioxidant Enzyme Expression in the Selective Cyototoxic Response of Glioma Cell to γ-Linolenic Acid Supplementation, Free Radic. Biol. Med. 28, 1143–1156.
Harwood, J.L. (1996) Recent Advances in the Biosynthesis of Plant Fatty Acids, Biochim. Biophys. Acta 1301, 7–56.
Griffiths, G., Brechany, E.Y., Jackson, F.M., Christie, W.W., Stymne, S., and Stobart, K. (1996) Distribution and Biosynthesis of Stearidonic Acid in Leaves of Borago officinalis. Phytochemistry 43, 381–386.
Gill, I., and Valivety, R. (1997) Polyunsaturated Fatty Acids, Part 1: Occurrence, Biological Activities and Applications, Trends Biotechnol. 15, 401–409.
Napier, J.A., Michaelson, L.V., and Stobart, A.K. (1999) Plant Desaturases: Harvesting the Fat of the Land, Curr. Opin. Plant Biol. 2, 123–127.
Sakuradani, E., Kobayashi, M., and Shimizu, S. (1999) Δ6-Fatty Acid Desaturase From an Arachidonic Acid-Producing Mortierella Fungus. Gene Cloning and Its Heterologous Expression in a Fungus, Aspergillus, Gene 238, 445–453.
Huang, Y.-S., Chaudhary, S., Thurmond, J.M., Bobik, E.G., Yuan, L., Chan, G.M., Kirchner, S.J., Mukerji, P., and Knutzon, D.S. (1999) Cloning of Δ12- and Δ6-Desaturases from Mortierella alpina and Recombinant Production of γ-Linolenic Acid in Saccharomyces cerevisiae, Lipids 34, 649–659.
Murphy, D.J. (1995) The Use of Conventional and Molecular Genetics to Produce New Diversity in Seed Oil Composition for the Use of Plant Breeders—Progress, Problems and Future Prospects, Euphytica 85, 433–440.
Reddy, A.S., and Thomas, T.L. (1996) Expression af a Cyanobacterial Δ6-Desaturase Gene Results in γ-Linolenic Acid Production in Transgenic Plants, Nat. Biotechnol. 14, 639–642.
López Alonso, D., and García Maroto, F. (2000) Plants as ‘Chemical Factories’ for the Production of Polyunsaturated Fatty Acids, Biotechnol. Adv. 18, 481–497.
Sayanova, O., Smith, M.A., Lapinskas, P., Stobart, A.K., Dobson, G., Christie, W.W., Shewry, P.R., and Napier, J.A. (1997) Expression of a Borage Desaturase cDNA Containing an N-Terminal Cytochrome b5 Domain Results in the Accumulation of High Levels of Δ6-Desaturated Fatty Acids in Transgenic Tobacco, Proc. Nat. Acad. Sci. USA 94, 4211–4216.
Guil-Guerrero, J.L., Gómez-Mercado, F., García-Maroto, F., and Campra-Madrid, P. (2000) Occurrence and Characterization of Oils Rich in γ-Linolenic Acid Part I: Echium Seeds from Macaronesia, Phytochemistry 53, 451–456.
Guil-Guerrero, J.L., Gómez-Mercado, F., Rodríguez-García, I., Campra-Madrid, P., and García-Maroto, F. (2001) Occurrence and Characterization of Oils Rich in γ-Linolenic Acid (III): The Taxonomical Value of the Fatty Acids in Echium (Boraginaceae). Phytochemistry 58, 117–120.
Hoekema, A., Hirsch, P.R., Hooykaas, P.J.J., and Schilperoot, R.A. (1983) A Binary Plant Vector Strategy Based on Separation of Vir-and T-Region of the Agrobacterium tumefaciens Ti-Plasmid, Nature 303, 179–180.
Schultz, L.D., Hofmann, K.J., Mylin, L.M., Montgomery, D.L., Ellis, R.W., and Hopper, J.E. (1987) Regulated Overproduction of the GAL4 Gene Product Greatly Increases Expression from Galactose-Inducible Promoters on Multi-Copy Expression Vectors in Yeast, Gene 61, 123–133.
Mylin, L.M., Hofmann, K.J., Schultz, L.D., and Hopper, J.E. (1990) Regulated GAL4 Expression Cassette Providing Controllable and High-Level Output from High-Copy Galactose Promoter in Yeast, Meth. Enzymol. 185, 297–309.
Ochman, H., Medhora, M., Garza, D., and Hartl, D.L. (1990) Amplification of Flanking Sequences by Inverse PCR, in PCR Protocols: A Guide to Methods and Applications (Innis, M.A., Gelfan, D.H., Sninsky, J.J., and White, T.J., eds.), pp. 219–227, Academic Press, London.
Thompson, J.D., Higgins, D.G., and Gibson, T.C. (1994) CLUSTALW: Improving the Sensitivity of Progressive Multiple Sequence Alignment Through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice, Nucl. Acids Res. 22, 4673–4680.
Saitou, N., and Nei, M. (1987) The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees, Mol. Biol. Evol. 4, 406–425.
Page, R.D.M. (1996) TREEVIEW: An Application to Display Phylogenetic Trees on Personal Computers, Comput. Appl. Biosci. 12, 357–358.
Taylor, B., and Powel, A. (1982) Isolation of Plant DNA and RNA, Focus 4, 4–6.
Chang, S., Puryear, J., and Cairney, J. (1993) A Simple and Efficient Method for Isolating RNA from Pine Trees, Plant Mol. Biol. Rep. 11, 113–116.
Shih, M.C., Lazan, G., and Goodman, H.M. (1986) Evidence in Favor of the Symbiotic Origin of Chloroplasts: Primary Structure and Evolution of Tobacco Glyceraldehyde-3-phosphate Dehydrogenases, Cell 47, 73–80.
Guerineau, F. (1995) Tools for Expressing Foreign Genes in Plants, Methods Mol. Biol. 49, 1–32.
Bevan, M. (1984) Binary Agrobacterium Vectors for Plant Transformation, Nucl. Acids Res. 12, 8711–8721.
Horsch, R.B., Fry, J.E., Hoffmann, N.L., Wallroth, M., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985) A Simple and General Method for Transferring Genes into Plants, Science 227, 1229–1231.
Ito, H., Fukuda, K., Murata, K., and Kimura, A. (1983) Transformation of Intact Yeast Cells Treated with Alkali Cations, J. Bacteriol. 153, 163–168.
Cesareni, G., and Murray, A.H. (1987) Plasmid Vectors Carrying the Replication Origin of Filamentous Single-Stranded Phages, in Genetic Engineering (Setlow, J.K., ed.), Vol. 9, pp. 135–154, Plenum Press, New York.
Rodríguez-Ruiz, J., Belarbi, E.-H., García Sánchez, J.L., and López Alonso, D. (1998) Rapid Simultaneous Lipid Extraction and Transesterification for Fatty Acid Analyses, Biotechnol. Tech. 12, 689–691.
Napier, J.A., Sayanova, O., Sperling, P., and Heinz, E. (1999) A Growing Family of Cytochrome b5-Domain Fusion Proteins, Trends Plant Sci. 4, 2–4.
Sperling, P., Zähringer, U., and Heinz, E. (1998) A Sphingolipid Desaturase from Higher Plants. Identification of a New Cytochrome b5 Fusion Protein, J. Biol. Chem. 273, 28590–28596.
Cahoon, F.B., Lindqvist, Y., Schneider, G., and Shanklin, J. (1997) Redesign of Soluble Fatty Acid Desaturases from Plants for Altered Substrate Specificity and Double Bond Position, Proc. Natl. Acad. Sci. USA 94, 4871–4877.
Sayanova, O., Davies, G.M., Smith, M.A., Griffith, G., Stobart, A.K., Shewry, P.R., and Napier, J.A. (1999) Accumulation of Δ6-Unsaturated Fatty Acids in Transgenic Tobacco Plants Expressing a Δ6-Desaturated from Borago officinalis, J. Exp. Bot. 50, 1647–1652.
Stymne, S., and Stobart, A.K. (1986) Biosynthesis of γ-Linolenic Acid in Cotyledons and Microsomal Preparations of the Developing Seeds of Common Borage (Borago officinalis), Biochem. J. 240, 385–393.
Sayanova, O., Beaudoin, F., Libisch, B., Shewry, P., and Napier, J. (2000) Mutagenesis of the Borage Δ6 Fatty Acid Desaturase, Biochem. Soc. Trans. 28, 636–638.
Girke, T., Schmidt, H., Zähringer, U., Reski, R., and Heinz, E. (1998) Identification of a Novel Δ6-Acyl-Group Desaturase by Targeted Gene Disruption in Physcomitrella patens, Plant J. 15, 39–48.
Napier, J.A., Hey, S.J., Lacey, D.J., and Shewry, P.R. (1998) Identification of a Caenorhabditis elegans Δ6-Fatty-Acid-Desaturase by Heterologous Expression in Saccharomyces cerevisiae, Biochem J. 330, 611–614.
Cho, H.P., Nakamura, M.T., and Clarke, S.D. (1999) Cloning, Expression, and Nutritional Regulation of the Mammalian Δ-6 Desaturase, J. Biol. Chem. 274, 471–477.
Brosius, J. (1999) RNAs from All Categories Generate Retrosequences That May be Exapted as Novel Genes or Regulatory Elements, Gene 238, 115–134.
Sayanova, O., Napier, J., and Shewry, P.R. (1999) Δ6-Unsaturated Fatty Acids in Species and Tissues of the Primulaceae, Phytochemistry 52, 419–422.
Guil-Guerrero, J.L., García-Maroto, F., Campra-Madrid, P., and Gómez-Mercado, F. (2000) Occurrence and Characterization of Oils Rich in γ-Linolenic Acid Part II: Fatty Acids and Squalene from Macaronesian Echium Leaves, Phytochemistry 54, 525–529.
Hofmann, K., and Stoffel, W. (1993) TMbase—A Database of Membrane Spanning Proteins Segments, Biol. Chem. Hoppe Seyler 374, 166.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
García-Maroto, F., Garrido-Cárdénas, J.A., Rodríguez-Ruiz, J. et al. Cloning and molecular characterization of the Δ6-desaturase from two Echium plant species: Production of GLA by heterologous expression in yeast and tobacco. Lipids 37, 417–426 (2002). https://doi.org/10.1007/s1145-002-0910-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s1145-002-0910-6