Abstract
Purpose
The possibility of using chemical and microbial additives to enhance the phytoextraction of mercury (Hg) and arsenic (As) from a multi-contaminated soil could be very effective, leading to a significant saving in terms of time and costs of the reclamation. The aim of this study was to evaluate the efficacy of the addition of (i) thiosulfate and (ii) metal-tolerant bacteria isolated from the polluted soil having plant growth promotion (PGP) potential to perform As and Hg phytoextraction by Brassica juncea and Lupinus albus.
Materials and methods
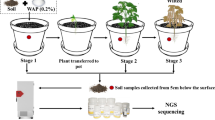
A collection of 13 bacterial isolates able to tolerate As and Hg was obtained from the contaminated soil, identified by partial 16S rRNA gene sequencing and tested in vitro for PGP activities. The most promising strains were further tested in vivo for the evaluation of plant growth ability and rhizocompetence on model plants. Pot experiments were conducted in microcosms, with polluted soil vegetated with B. juncea and L. albus. Ammonium thiosulfate and potassium dihydrogen phosphate were used as mobilizing agents, together with a bacterial consortium composed by the most promising PGP isolates.
Results and discussion
Thirteen indigenous metal-tolerant bacterial strains were isolated, and their in vitro characterization highlighted their great potential in assisting the phytoremediation process; most of them tolerated both trace elements and showed, at the same time, multiple PGP traits. The results were confirmed in vivo on model plants and lead to the selection of the most promising PGP strains to be applied in microcosm-scale phytoextraction experiments. Thiosulfate addition significantly increased the mobilization of both elements, promoting bioavailability and phytoextraction. When a selected bacterial consortium was supplemented in addition to thiosulfate, the efficacy of the phytoaccumulation was increased up to 85 % for As and up to 45 % for Hg.
Conclusions
The use of the common fertilizer thiosulfate appeared to have great potential in phytoextraction practices since it was able to facilitate the uptake by plants of both Hg and As. Moreover, the application of a consortium of indigenous PGP bacteria (PGPB) produced a further positive effect on the plant biomass, supporting and enhancing the phytoextraction strategy, thus demonstrating their potential in a microbe-assisted phytoremediation intervention.





Similar content being viewed by others
References
Aafi NE, Brhada F, Dary M, Maltouf AF, Pajuelo E (2012) Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant rhizobacterium Serratia sp. MSMC541. Int J Phytorem 14(3):261–274
AMAP/UNEP (2013) Technical background report for the global mercury assessment 2013. Arctic monitoring and assessment
Arenskötter M, Bröker D, Steinbüchel A (2004) Biology of the metabolically diverse genus gordonia. Appl Environ Microb 6:3195–3204
Barbafieri M, Japenga J, Romkens P, Petruzzelli G, Pedron F (2013) Protocols for applying phytotechnologies in metal-contaminated soils. In: Gupta DK (ed) Plant-based remediation processes. Springer, Berlin, Heidelberg, pp 19–37
Barber SA (1984) Soil nutrient bioavailability. John Wiley & Sons, NY
Barbosa-Jefferson VL, Zhao FJ, McGrath SP, Magan N (1998) Thiosulphate and tetrathionate oxidation in arable soils. Soil Biol Biochem 30:553–559
Beauregarda PB, Chaib Y, Vlamakisa H, Losickb R, Koltera R (2013) Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci U S A 110(17):1621–1630
Brick JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Cappuccino JC, Sherman N (1992) Microbiology: a laboratory manual, 3rd ed. Benjamin/Cummings Publishing Company, New York 125–179
Duan G, Liu W, Chen X, Hu Y, Zhu Y (2013) Association of arsenic with nutrient elements in rice plants. Metallomics 5:784–792
Fukushi K, Sverjensky DA (2007) A surface complexation model for sulfate and selenate on iron oxides consistent with spectroscopic and theoretical molecular evidence. Geochim Cosmochim Ac 71:1–24
Fumagalli P, Comolli R, Ferre C, Ghiani A, Gentili R, Citterio S (2014) The rotation of white lupin (Lupinus albus L.) with metal-accumulating plant crops: a strategy to increase the benefits of soil phytoremediation. J Environ Manag 145:35–42
Gadd GM (2000) Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol 11:271–279
Garelick H, Jones H, Dybowska A, Valsami-Jones E (2008) Arsenic pollution sources. In: Garelick H, Jones H (eds) Arsenic pollution and remediation: an international perspective. Springer, New York, pp 17–60
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis. Part 1 Physical and mineralogical methods, 2nd edn. Agronomy Monograph No. 9, American Society of Agronomy/Soil Science Society of America, Madison, WI., pp 383–411
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its by products. As J Energy Env 6(04):214–231
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374
Glick BR, Patten CL, Holguin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London, UK
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Gutiérrez-Ginés MJ, Hernández AJ, Pérez-Leblic MI, Pastor J, Vangronsveld J (2014) Phytoremediation of soils co-contaminated by organic compounds and heavy metals: bioassays with Lupinus luteus L. and associated endophytic bacteria. J Environ Manage 143:197–207
Islam MR, Madhaiyan M, Deka Boruah HP, Yim W, Lee G, Saravanan VS, Fu Q, Hu H, Sa T (2009) Characterization of plant growth-promoting traits of free-living diazotrophic bacteria and their inoculation effects on growth and nitrogen uptake of crop plants. J Microbiol Biotechnol 12:13–22
Jarrell WM, Beverly RB (1981) Dilution effect in plant nutrition studies. Adv Agron 34:197–224
Jiang C, Sheng X, Qian M, Wang Q (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72:154–164
Kumar VK, Patra DD (2013) Influence of nickel and cadmium resistant PGPB on metal accumulation and growth response of Lycopersicum esculentum plants grown in fly ash amended soil. Water Air Soil Poll 224:1357
Liba CM, Ferrara FIS, Manfio GP, Fantinatti-Garboggini F, Albuquerque RC, Pavan C, Ramos PL, Moreira-Filho CA, Barbosa HR (2006) Nitrogen-fixing chemo-organotrophic bacteria isolated from Cyanobacteria-deprived lichens and their ability to solubilize phosphate and to release amino acids and phytohormones. J Appl Microbiol 101–5:1076–1086
Loper JE, Scroth MN (1986) Influence of bacterial sources on indole-3 acetic acid on root elongation of sugarbeet. Phytopathology 76:386–389
Meers E, Tack FMG, Van Slycken S, Ruttens A, Du Laing G, Vangronsveld J, Verloo MG (2008) Chemically assisted phytoextraction: a review of potential soil amendments for increasing plant uptake of heavy metals. Int J Phytoremediation 10:390–414
Milagres AMF, Machuca A, Napoleão D (1999) Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J Microbiol Meth 37–1:1–6
Moreno FN, Anderson CWN, Stewart RB, Robinson BH (2004) Phytoremediation of mercury-contaminated mine tailings by induced plant-mercury accumulation. Environ Pract 6:165–175
Moreno FN, Anderson CWN, Stewart RB, Robinson BH, Ghomshei M, Meech JA (2005) Induced plant uptake and transport of mercury in the presence of sulphur-containing ligands and humic acid. New Phytol 166:445–454
Morris CE, Monier JM (2003) The ecological significance of biofilm formation by plant-associated bacteria. Annu Rev Phytopathol 41:429–453
Myneni SCB, Traina SJ, Waychunas GA, Logan TJ (1998) Vibrational spectroscopy of functional group chemistry and arsenate coordination in ettringite. Geochim Cosmochim Acta 62:3499–3514
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Sparks DL (ed) Methods of soil analysis. Part 3 Chemical Methods. Soil Science Society of America Book Series, Soil Science Society of America Inc, Madison, USA., pp 961–1010
Nielsen P, Sørensen J (1997) Multi-target and medium-independent fungal antagonism by hydrolitic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol Ecol 22–3:183–192
Pedron F, Petruzzelli G, Barbafieri M, Tassi E, Ambrosini P, Patata L (2011) Mercury mobilization in a contaminated industrial soil for phytoremediation. Commun Soil Sci Plan 42:2767–2777
Pedron F, Petruzzelli G, Barbafieri M, Tassi E (2013) Remediation of a mercury-contaminated industrial soil using bioavailable contaminant stripping. Pedosphere 23(1):104–110
Petruzzelli G, Pedron F, Rosellini I, Barbafieri M (2013) Phytoremediation towards the future: focus on bioavailable contaminants. In: Gupta DK (ed) Plant-based remediation processes. Springer, Berlin, Heidelberg, pp 273–289
Petruzzelli G, Pedron F, Rosellini I (2014) Effects of thiosulfate on the adsorption of arsenate on hematite with a view to phytoextraction. Res J Environ Earth Sci 6(6):326–332
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574
Romheld V, Marschner H (1986) Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr 2:155–204
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100–8:4927–4932
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Salisbury FB (1994) The role of plant hormones. In: Wilkinson RE (ed) Plant–environment interactions., pp 39–81
Santaella C, Schue M, Berge O, Heulin T, Achouak W (2008) The exopolysaccharide of Rhizobium sp. YAS34 is not necessary for biofilm formation on Arabidopsis thaliana and Brassica napus roots but contributes to root colonization. Environ Microbiol 10(8):2150–2163
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Shahab S, Ahmed N, Khan NS (2009) Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. Afr J Agric Res 4(11):1312–1316
Sparks DL (1998) Methods of soil analysis. Part 3. Chemical Methods, Soil Science Society of America Book Series. Soil Science Society of America, Madison, WI
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In: Sparks DL (ed) Methods of soil analysis. Part 3 Chemical Methods. Soil Science Society of America Book Series, Soil Science Society of America Inc, Madison, USA., pp 1201–1230
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A 101:11030–11035
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tassi E, Pedron F, Barbafieri M, Petruzzelli G (2004) Phosphate-assisted phytoextraction in As-contaminated soil. Eng Life Sci 4:341–346
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (ed) Methods of soil analysis. Part 3 Chemical Methods, Soil Science Society of America Book Series, Soil Science Society of America Inc., Madison, USA., pp 475–490
USEPA (1998) SW-846 Method 7473. Mercury in solids and solutions by thermal decomposition, amalgamation and atomic absorption spectrophotometry. Method 7473, U.S. EPA, Washington, DC
Wang J, Feng X, Anderson CWN, Wang H, Wang L (2014) Thiosulphate-induced mercury accumulation by plants: metal uptake and transformation of mercury fractionation in soil—results from a field study. Plant Soil 375:21–33
Wani PA, Khan MS, Zaidi A (2007) Plant growth-promoting potentials, and metal solubilization by Bacillus sp. isolated from alluvial soil. Curr Microbiol 54(3):237–243
Weaver PK, Wall JD, Guest H (1975) Characterization of Rhodopseudomonas capsulata. Arch Microbiol 105:207–216
Wu SC, Cheung KC, Luo YM, Wong MH (2006) Effects of inoculation of plant growth-promoting Rhizobacteria on metal uptake by Brassica juncea. Environ Pollut 40:124–135
Acknowledgments
The experimental work carried out at ISE-CNR was fully funded by Syndial s.p.a. ER was supported by Università degli Studi di Milano, DeFENS, the European Social Found, and Regione Lombardia (contract “Dote Ricerca”). RM was financially supported by the project BIOGESTECA no. 15083/RCC “Fondo per la Promozione di Accordi Istituzionali” (Regione Lombardia, Italy). We thank Francesco Rodriguez for the bioinformatic support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jaco Vangronsveld
Rights and permissions
About this article
Cite this article
Franchi, E., Rolli, E., Marasco, R. et al. Phytoremediation of a multi contaminated soil: mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J Soils Sediments 17, 1224–1236 (2017). https://doi.org/10.1007/s11368-015-1346-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1346-5