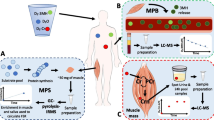
Abstract
Validated diagnostics of skeletal muscle vitality could benefit clinical and basic science in terms of mechanistic insights and in determining the efficacy of interventions, e.g. exercise/pharmaceuticals/nutrients. We recently developed a Combined Oral Assessment of Muscle (COSIAM) that can be used to simultaneously quantify whole-body muscle mass (WBMM), muscle protein synthesis (MPS) and muscle protein breakdown (MPB). Here, we aimed to establish, in a cross-sectional fashion, links between COSIAM parameters and established aspects of muscle function. We recruited 37 healthy older adults (male (M):female (F) (21/16); 72 ± 5 y)) into a 3-day trial. Subjects consumed D3-creatine (D3-Cr dilution to assess WBMM), D2O (MPS by incorporation of alanine) and D3-3-methylhistidine (D3-MH dilution to assess MPB). A biopsy at day 3 was used to determine MPS, and blood/urine samples were collected to determine D3-Cr/D3-MH dilution for WBMM and MPB. Physiological measures of muscle mass (e.g. DXA/ultrasound) and function (e.g. handgrip strength, maximum voluntary contraction (MVC), one-repetition maximum (1-RM)) were ascertained. A stepwise linear regression approach was used to address links between facets of COSIAM (MPS, MPB, WBMM) and muscle physiology. Despite expected differences in muscle mass, there were no significant differences in MPS or MPB between sexes. WBMM as measured using D3-Cr positively correlated with DXA-derived lean body mass (LBM) and appendicular LBM (ABLM). Stepwise linear regression was used to assess which combination of MPS, MPB, D3-Cr and absolute synthesis rate (ASR) best predicted physiological measures of muscle health in these older adults. D3-Cr WBMM alone was the best predictor of handgrip, 1RM and MVC, and outperformed more traditional measures of muscle mass by DXA. The COSIAM approach substantiates D3-Cr as a robust biomarker of multiple muscle physiology health biomarkers. Future work using COSIAM should focus upon how and which parameters it can inform upon in relation to disease progression and the efficacy of interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Declines in muscle mass and function are hallmarks of ageing culminating in the manifestation of sarcopenia and frailty [20]. As a result, those living with sarcopenia face an increasing risk of falls, fractures and loss of independence [21]—engendering a major social and economic burden in the face of the sustained spike in longevity across many parts of the world. Muscle mass is metabolically regulated by proteostasis, ultimately reflected in the longer-term balance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB). As such, the measurement of these facets alongside muscle mass itself remains a cornerstone to understanding the basis of age-related muscle loss, but also the efficacy of mitigation strategies, such as exercise [12], nutrition [3, 6] and pharmaceuticals [8]. Finally, iterations of sarcopenia diagnostic criteria, e.g., by the EWGSOP [1], have established an ICD-10 code for sarcopenia, which may encourage the pharmaceutical industry to re-visit the impact of drugs and value of biomarkers relating to this progressive condition.
The principal methods to assess “muscle health” (or vitality) in the literature centre around measures of whole-body or regional muscle mass, muscle function and protein turnover, i.e. MPS and MPB. We recently developed a minimally invasive Combined Oral Stable Isotope Assessment of Muscle (COSIAM) approach where we provide a protocol capable of simultaneous quantification of muscle mass (via a deuterated creatine dilution technique [5, 16, 17]), muscle protein synthesis (via D2O [2, 18, 19]) and muscle protein breakdown (via deuterated 3-methylhistidine dilution [5, 14]). The purpose of setting up this approach was to offer researchers a single protocol to determine these variables in older, clinical (vs. healthy) and difficult to study populations, and also in response to candidate interventions. Nonetheless, from a cross-sectional perspective, an important next-step is to assess how COSIAM facets link to broader physiological biomarkers of muscle health (i.e. mass/functional characteristics).
In our previous paper [5], we conducted a pilot study in a small number (N = 10) of subjects aimed at validation of the COSIAM approach from a purely technical and logistical perspective. The aim of the present study was to expand this pilot work and determine relationships between COSIAM facets and cross-sectional muscle health parameters in a larger group of older men and women. We sought to determine which facets of COSIAM relate to functional muscle health parameters (e.g. handgrip strength, leg strength, muscle mass by DXA imaging), on a cross-sectional basis in older individuals of both sexes.
Methods
Participant recruitment, ethics and study data collection
This work utilises and extends data collected as part of previously published work [5]. This study was reviewed and approved by the University of Nottingham Faculty of Medicine & Health Sciences Research Ethics Committee (A08122015) and was performed in accordance with the Declaration of Helsinki (2013). Thirty-seven healthy older (M:F (21/16); 72 ± 5 y)) adults were recruited. All participants were screened by means of a medical questionnaire, physical examination and resting ECG. Participants were excluded if they had a BMI > 35 kg·m2, active cardiovascular disease, cerebrovascular disease, respiratory disease, metabolic disease, inflammatory bowel or renal disease, active malignancy, any musculoskeletal or neurological disorders, any signs of clotting dysfunction, or any recent (within 6 months) steroid treatment, or if on hormone replacement therapy. All participants were informed of the purpose of the study and of all the risks and procedures involved, before providing their written, informed consent to participate.
On the first study day (day 1), participants were asked to attend the unit at 8:30 am for a whole-body DXA scan, having fasted overnight. They next provided a baseline saliva sample (to measure baseline body water enrichment); a baseline blood sample was then taken to measure background blood/plasma alanine labelling prior to D2O exposure, and baseline urine sample collected to measure D3-creatinine background enrichment. Muscle architecture was measured by ultrasound before performing any muscle function assessments. Participants then performed the Short Physical Performance Battery (SPPB). Leg extensor (unilateral one-repetition maximum (1-RM) and maximal voluntary contraction (MVC) for leg extension) and handgrip strength (using handgrip dynamometer) were also measured. Participants were then given three 50-ml aliquots of 70 atom percent (AP) D2O to drink, each taken 25 min apart (to minimise the risk of side effects such as dizziness or nausea that are sometimes reported after D2O consumption due to disturbance of specific gravity within the vestibular fluid [10]). Thirty milligrammes D3-Cr was included in the final 50-ml aliquot of D2O. Two hours after consumption of the final D2O aliquot, participants provided another saliva sample, to be used to determine the plateau D2O labelling in body water.
Prior to leaving the laboratory, participants were provided with a container to collect all urine for 24 h, a tube for a spot urine sample at 48 h and three tubes for saliva samples, collected at 12, 24 and 48 h after the final D2O aliquot was consumed. The urine samples were used to measure D3-creatine spill-over, urinary D3-creatinine enrichment and the size of the creatine pool, respectively. Saliva was collected to monitor the decay in D2O over time to determine precursor alanine labelling. Participants were also given 10 mg of D3-3MH dissolved in 50 ml of water to take home and store refrigerated. Participants were instructed to consume the D3-3MH immediately 48 h after the urine and saliva samples were collected.
Participants returned to the laboratory 72 h after consumption of the final D2O aliquot which was ~ 20–22 h after the D3-3MH consumption, again having fasted overnight. At this visit, hourly bloods were collected over 6 h (between 22 and 28 h after consumption of the D3-3MH dose), with further spot urine and saliva samples collected at 72 h after D3-3MH consumption. A muscle biopsy from the m. vastus lateralis (VL) was also taken at the start of this visit (72 h) under local anaesthetic (1% lidocaine). The Bard micro-needle biopsy technique (50% length of VL, mid-belly) was used for the first 8 participants, with the conchotome approach [7]. Participants were not fasted during the blood sampling period, but given a standardised light meal (sandwich, crisps, yoghurt and drink) at the mid-point of the blood collections. Figure 1 (taken from [5] illustrates the protocol.
Muscle functional tests
MVC was measured as previously described [2] with isometric contractions conducted in a sitting position using an isokinetic dynamometer at a 70° knee joint angle (Isocom Iskokinetics, Inc. De Queen, AR, USA). Participants were seated in the dynamometer chair and secured into position using a chest strap. Contractions lasted 5 s, with a 30-s rest period between contractions. Unilateral leg extension 1-RM was assessed on the dominant leg (ISO Leg Extension, Leisure Lines, Leicestershire, UK). After the procedure was explained, subjects performed a light warm up to ensure familiarity whilst avoiding fatigue; 1-RM was then achieved in as few repetitions as possible, with a maximum of 5 repetitions. The first repetition was estimated at 50% 1-RM and then increased until participants could not complete controlled contraction throughout the range of motion, with 3-min in-between attempts. Handgrip strength was assessed using a digital handgrip dynamometer (Grip D 5401; Takei Scientific Instruments Ltd, Japan) in each arm. The width of the dynamometer was adjusted for each participant, with participants instructed to stand upright with the arm fully extended along the body, maintaining an ∼5-cm gap between the wrist and the hip or upper leg so that the hand was not rested against the body. Participants were then instructed to squeeze against the handle as hard possible for 3 s. Grip strength was measured three times and recorded in kilogrammes to the nearest 0.1 kg. For the purpose of this study, the best of three attempts was included in further analysis from the stronger arm.
Muscle architecture, DXA-derived mass
Muscle architecture was measured as described previously [2]. Briefly, the architectural parameter muscle thickness (MT), fascicle length (Lf) and pennation angle (θ) were quantified, by the same investigator from ultrasound scans using ImageJ 1.42q software (National Institutes of Health, Bethesda, MD). The visible portion of the Lf was assessed directly using this software with MT measured as the distance between the superficial and deep aponeuroses. θ was measured at the intersection between fascicles and the deep tendon aponeurosis. DXA-derived thigh fat-free mass (TFFM) was determined from the lowest point of the ischium to the mid-point of the patella.
Statistical analyses
Here we aimed to assess whether any of the metabolic variables (MPS, MPB, D3-creatine, ASR) determined from the COSIAM approach, either individually or in combination with each other, could accurately predict our physiological outcomes (Handgrip, MVC, 1-RM, WBMM). Multivariate linear regression was performed using base R (https://cran.r-project.org/), with best subset regression implemented using the leaps package (https://cran.r-project.org/web/packages/leaps/index.html). Performance of each model was assessed using a combination of root mean squared error (RMSE) and R squared (R2) using the performance R Package (https://CRAN.R-project.org/package=performance), with those variables providing the lowest RMSE and highest R2 deemed to provide the best predictive power. All processing was performed in R (with scripts available from the authors upon request), and all plots were constructed in R using ggplot2 or GraphPad Prism v.9 (La Jolla, CA, USA). To assess sex differences in MPS and MPB, unpaired t-tests were performed following assessment for normal distribution of the data, and correlations were assessed using Pearson’s product moment correlation coefficient. The alpha level of significance for all measures was set at p < 0.05.
Results
Muscle protein synthesis (MPS), muscle protein breakdown (MPB) and muscle mass
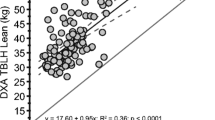
Mean MPS and MPB values as measured using the COSIAM approach are depicted in Fig. 2A and B. There were no differences observed between sexes, and measured rates fell within expected values for each, thereby confirming the accuracy and reproducibility of the COSIAM approach. Muscle mass as measured using D3-creatine showed significant positive correlations with both LBM and ALBM (Fig. 2C), with good agreement observed in absolute values between D3-creatine and ALBM.
Sex differences for all COSIAM facets in older adults (n = 21 men, 16 women). A Muscle protein synthesis (MPS) as measured using D2O, B muscle protein breakdown (MPB) as measured via tracer dilution (k) rate of D3-3-Methylhistidine and C muscle mass as estimated using the D3-creatine dilution approach. Abbreviations: FSR, fractional synthetic rate; DXA, dual-energy absorptiometry
COSIAM as a measure of muscle health
To assess the relationship between facets of the COSIAM approach (MPS, MPB, ASR and D3-creatine) and established measures of muscle health (handgrip strength, 1-RM and MVC), multivariate linear regression was used. Due to missing data for the key measures of 1-RM and MVC (as a result of unavailable equipment) for a small number of participants, linear regression was only possible to perform for n = 29. The aim of this approach was to assess which variable or group of variables provided the most optimal predictive model for each of the individual physiological measure of muscle health. This was performed using a stepwise or best subset regression approach implemented in the leaps R package, whereby a separate least squares regression model was fitted to each combination of the predictor variables: MPS, MPB, ASR and D3-creatine. To determine the performance of each of the models for predicting each of our outcome variables, k-fold cross validation was performed for each subset regression model, with the one providing the lowest prediction error determined to be the best predictive model. The magnitude of effect for each predictor variable in the full model is shown in Fig. 3. Using the best subset approach, D3-creatine alone was found to be the best predictor for each of the physiological measures of handgrip, 1R-M and MVC (see Table 1); the effect plots for each optimal model can be observed in Fig. 3, with D3-creatine showing a strong positive relationship with each outcome variable.
Forest plots showing the magnitude of effects for each predictor variable: MPS, MPB, ASR and D3-creatine, on each muscle health outcome variable (n = 29): handgrip strength, 1-RM and MVC, when each predictor variable is included in the regression model. Abbreviations: MPS-d, muscle protein synthesis per day; MPB, muscle protein breakdown; ASR, absolute synthetic rate; 1-RM, one-repetition maximum; MVC, maximum voluntary contraction
D3-creatine as highlighted in Fig. 2C has a strong relationship with DXA-derived WBMM. Therefore, to determine whether D3-creatine performed as a better predictor of each physiological outcome than DXA-derived measures, linear regression models of D3-creatine and DXA-derived lean mass were constructed and predictive performance of both models directly compared. Performance metrics for each model are provided in Table 2. For all three physiological outcomes of handgrip, 1RM and MVC, D3-creatine performed better at predicting outcomes than DXA-derived lean mass, as evidenced by the greater R squared (and adjusted R squared) and lower RMSE for D3-creatine.
Discussion
The present study follows on from prior pilot work in which we developed a protocol fit for purpose to simultaneously quantify WBMM, MPS and MPB using the COSIAM approach. In the face of increasing difficulties in deploying alternative techniques to quantify these facets of muscle health (outlined below), we sought to develop a more practicable, less invasive and more widely applicable method. For example, prohibitively expensive imaging methods such as MRI are often use for WBMM whilst stable isotopically labelled amino acids by I.V infusions for quantifying MPS are limited by their short-term nature, need for pharmacy QC, costs and ethical issues. Similarly quantifying MPB is a notoriously challenging dynamic measurement where A-V balance models, for instance, rely on the detection of negligible tracer dilution and accurate blood flow measurements across a limb bed.
The COSIAM approach that we developed [5] indicated accurate measures of diurnal MPS and MPB in accordance with our and others’ prior research [9, 19]. In terms of sex, despite no statistical differences, women tended to exhibit numerically higher rates of MPS than older men. Differences in acute (i.e. over a few hours) MPS were previously reported between older men and women [15], with it being proposed that a greater basal (i.e. postabsorptive) MPS which operates over a majority of the diurnal cycle may explain slower age-related muscle loss in women than in men. Our present results in showing a trend (but not statistical significance) underline at this stage, if nothing else, potential sexual dimorphism of protein metabolism in ageing and the need to consider sex differences in the wider field of age-related sarcopenia as necessary. Also, that integrated measures of MPS over day(s) may not reflect those of a single diurnal fasted-fed cycle. Finally, our work is also in agreement with others showing that whilst D3-Cr measures of muscle mass correlate with DXA, DXA markedly underestimates WBMM, which is intuitive given that a major limitation of DXA is its inability to distinguish between lean tissue and muscle tissue per se [13].
The principal objective of this study was to determine links between facets of COSIAM and that of more established biomarkers of muscle mass and function. To address this, rather than focusing upon myriad individual correlations, we undertook multivariate linear regression to assess which variable—or groups of variables—provided the most optimal model for predicting each individual physiological measure of muscle vitality. In this cross-sectional trial, we report that D3-Cr-derived WBMM was able to best predict core markers of muscle health, including handgrip strength (used in much epidemiology muscle research [11]) and knee-extensor 1-RM and MVC, commonly used as muscle strength assessments in muscle physiology circles. The notion of D3-Cr-derived WBMM being superior to other imaging techniques has been established, with low D3-Cr-derived muscle mass being identified as a novel risk factor for clinically meaningful outcomes in older men [4], and with associations of D3-Cr being more closely associated with measures of physical function in older women, than DXA [22]. Our work corroborates that even when compared to MPS and MPB, cross-sectionally D3-Cr-derived WBMM most closely relates to muscle function. It is likely that this is due to D3-Cr providing a direct readout of contractile mass (Fig. 4) .
We also provide readers with a matrix of relationships between each of the variables that were measured in this trial (Fig. 5). This matrix illustrates the strengths of some of these already well-described links between variables, for instance, between handgrip strength and sex, but also more novel observations such as those between MPS rates corrected for muscle mass (absolute synthesis rate (ASR)), and measures of muscle functional capacity such as 1-RM and MVC. This may suggest a potential application of measuring MPS for predicting functional outcomes when corrected for muscle mass; however, further validatory work is needed to confirm this. Finally, this correlation matrix further highlights the strength of relationships between D3-Cr-derived WBMM and DXA/muscle function outcomes, and hence its power for potentially predicting these outcomes as evidenced by this work.
Correlation matrix of relationships amongst all measured variables (n = 29). Abbreviations: MPS-d, muscle protein synthesis per day; ASR, absolute synthetics rate; ASMI, appendicular skeletal mass index; BMC, bone mineral content; BMD, bone mineral density; RM, repetition maximum; MVC, maximum voluntary contraction; Vl, vastus lateralis; BMI, body mass index; MPB, muscle protein breakdown
Finally, we acknowledge potential limitations of COSIAM, which include the whole body (non-regional) nature of the measure of muscle mass,; that assumptions are based on a standardised creatine pool size (although with a muscle biopsy, direct creatine measures can be made in a given population); that whilst somewhat minimally invasive, this approach as of now still requires a muscle biopsy; and that diet and exercise behaviours could impact outcomes and could/should be monitored to assess any study- or population-specific effects, in future. Despite this, the COSIAM method is a minimally invasive and valid method to assess WBMM, MPS and MPB simultaneously, offering much potential in clinical and difficult to study populations, e.g. care home residents and patient populations at risk of muscle wasting. Whilst the present work cements D3-Cr as a robust cross-sectional biomarker of muscle physiology, future interventional work will establish how protein turnover interacts with D3-Cr-derived measures of WBMM in situations of change, e.g. during acute illness, chronic disease or in response to exercise/drug or other interventions.
References
Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512–514. Available at: https://pubmed.ncbi.nlm.nih.gov/27891296/ [Accessed December 16, 2021].
Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol. 2016;594:7399–417.
Bukhari SSI, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, Kobayashi H, Greenhaff PL, Smith K, Atherton PJ. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol - Endocrinol Metab. 2015;308:E1056–65.
Cawthon PM, Blackwell T, Cummings SR, Orwoll ES, Duchowny KA, Kado DM, Stone KL, Ensrud KE, Cauley JA, Evans WJ. Muscle mass assessed by the D3-creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community-dwelling older men. J Gerontol A Biol Sci Med Sci. 2020;76:123–30.
Cegielski J, Wilkinson DJ, Brook MS, Boereboom C, Phillips BE, Gladman JFR, Smith K, Atherton PJ. Combined in vivo muscle mass, muscle protein synthesis and muscle protein breakdown measurement: a ‘Combined Oral Stable Isotope Assessment of Muscle (COSIAM)’ approach. GeroScience. 2021;43:2653–65.
Deane CS, Wilkinson DJ, Phillips BE, Smith K, Etheridge T, Atherton PJ. “Nutraceuticals” in relation to human skeletal muscle and exercise. Am J Physiol Endocrinol Metab. 2017. https://doi.org/10.1152/ajpendo.00230.2016.
Dietrichson P, Coakley J, Smith PE, Griffiths RD, Helliwell TR, Edwards RH. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatry. 1987;50:1461–7.
Gharahdaghi N, Rudrappa S, Brook MS, Idris I, Crossland H, Hamrock C, Abdul Aziz MH, Kadi F, Tarum J, Greenhaff PL, Constantin-Teodosiu D, Cegielski J, Phillips BE, Wilkinson DJ, Szewczyk NJ, Smith K, Atherton PJ. Testosterone therapy induces molecular programming augmenting physiological adaptations to resistance exercise in older men. J Cachexia Sarcopenia Muscle. 2019. https://doi.org/10.1002/jcsm.12472.
Hector AJ, McGlory C, Damas F, Mazara N, Baker SK, Phillips SM. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018;32:265–75.
Jones PJ, Leatherdale ST. Stable isotopes in clinical research: safety reaffirmed. Clin Sci (Lond). 1991;80:277–80.
McGrath RP, Kraemer WJ, Al SS, Peterson MD. Handgrip strength and health in aging adults. Sport Med. 2018;48:1993–2000.
Phillips BE, Williams JP, Greenhaff PL, Smith K, Atherton PJ. Physiological adaptations to resistance exercise as a function of age. JCI Insight. 2017. https://doi.org/10.1172/jci.insight.95581.
Prado CMM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enter Nutr. 2014;38:940–53.
Sheffield-Moore M, Dillon EL, Randolph KM, Casperson SL, White GR, Jennings K, Rathmacher J, Schuette S, Janghorbani M, Urban RJ, Hoang V, Willis M, Durham WJ. Isotopic decay of urinary or plasma 3-methylhistidine as a potential biomarker of pathologic skeletal muscle loss. J Cachexia Sarcopenia Muscle. 2014;5:19–25.
Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoSOne. 2008;3:e1875-.
Stimpson SA, Leonard MS, Clifton LG, Poole JC, Turner SM, Shearer TW, Remlinger KS, Clark RV, Hellerstein MK, Evans WJ. Longitudinal changes in total body creatine pool size and skeletal muscle mass using the D3-creatine dilution method. J Cachexia Sarcopenia Muscle. 2013. https://doi.org/10.1007/s13539-013-0110-1.
Stimpson SA, Turner SM, Clifton LG, Poole JC, Mohammed HA, Shearer TW, Waitt GM, Hagerty LL, Remlinger KS, Hellerstein MK, Evans WJ. Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl-d3) dilution in rats. J Appl Physiol. 2012;112:1940–8.
Wilkinson DJ, Cegielski J, Phillips BE, Boereboom C, Lund JN, Atherton PJ, Smith K. Internal comparison between deuterium oxide (D2O) and L-[ring-13C6] phenylalanine for acute measurement of muscle protein synthesis in humans. Physiol Rep. 2015. https://doi.org/10.14814/phy2.12433.
Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff PL, Atherton PJ, Smith K. A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab. 2014. https://doi.org/10.1152/ajpendo.00650.2013.
Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–32.
Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:485–500. Available at: https://pubmed.ncbi.nlm.nih.gov/30993881/ [Accessed December 16, 2021].
Zhu K, Wactawski-Wende J, Ochs-Balcom HM, Lamonte MJ, Hovey KM, Evans W, Shankaran M, Troen BR, Banack HR. The association of muscle mass measured by D3-Creatine dilution method with dual-energy x-ray absorptiometry and physical function in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2021;76:1591–9.
Funding
This work and Jessica Cegielski were supported by a PhD studentship (RGS117347) from The Abbeyfield Research Foundation (charity number 1167685). This work was supported by the Medical Research Council (grant number MR/P021220/1) as part of the MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research awarded to the Universities of Nottingham and Birmingham and the National Institute for Health Research (NIHR), Nottingham Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the Medical Research Council, NHS, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the University of Nottingham Faculty of Medicine & Health Sciences Research Ethics Committee (A08122015) and was performed in accordance with the Declaration of Helsinki (2013). All participants provided written informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel J Wilkinson and Philip J Atherton are joint senior authors
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Cegielski, J., Brook, M.S., Phillips, B.E. et al. The Combined Oral Stable Isotope Assessment of Muscle (COSIAM) reveals D-3 creatine derived muscle mass as a standout cross-sectional biomarker of muscle physiology vitality in older age. GeroScience 44, 2129–2138 (2022). https://doi.org/10.1007/s11357-022-00541-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00541-3