Abstract
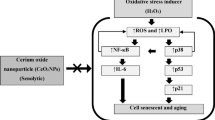
Human diploid fibroblasts (HDFs) exposed to subcytotoxic concentrations of oxidative or stressful agents, such as hydrogen peroxide, tert-butylhydroperoxide, or ethanol, undergo stress-induced premature senescence (SIPS). This condition is characterized by the appearance of replicative senescence biomarkers such as irreversible growth arrest, increase in senescence-associated β-galactosidase (SA β-gal) activity, altered cell morphology, and overexpression of several senescence-associated genes. Copper is an essential trace element known to accumulate with ageing and to be involved in the pathogenesis of some age-related disorders. Past studies using either yeast or human cellular models of ageing provided evidence in favor of the role of intracellular copper as a longevity modulator. In the present study, copper ability to cause the appearance of senescent features in HDFs was assessed. WI-38 fibroblasts exposed to a subcytotoxic concentration of copper sulfate presented inhibition of cell proliferation, cell enlargement, increased SA β-gal activity, and mRNA overexpression of several senescence-associated genes such as p21, apolipoprotein J (ApoJ), fibronectin, transforming growth factor β-1 (TGF β1), insulin growth factor binding protein 3, and heme oxygenase 1. Western blotting results confirmed enhanced intracellular p21, ApoJ, and TGF β1 in copper-treated cells. Thus, similar to other SIPS-inducing agents, HDF exposure to subcytotoxic concentration of copper results in premature senescence. Further studies will unravel molecular mechanisms and the biological meaning of copper-associated senescence and lead to a better understanding of copper-related disorder establishment and progression.





Similar content being viewed by others
References
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s Diseases. Curr Opin Chem Biol 12(2):222–228. doi:10.1016/j.cbpa.2008.02.019
Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI (1988) Human-skin fibroblasts invitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A 85(14):5112–5116
Borghouts C, Werner A, Elthon T, Osiewacz HD (2001) Copper-modulated gene expression and senescence in the filamentous fungus Podospora anserina. Mol Cell Biol 21(2):390–399
Borghouts C, Scheckhuber CQ, Stephan O, Osiewacz HD (2002) Copper homeostasis and aging in the fungal model system Podospora anserina: differential expression of PaCtr3 encoding a copper transporter. Int J Biochem Cell Biol 34(11):1355–1371
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brewer GJ (2010) Risks of copper and iron toxicity during aging in humans. Chem Res Toxicol 23(2):319–326
Chen Q, Ames BN (1994) Senescence-like growth arrest induced by hydrogen-peroxide in human-diploid fibroblast F65 cells. Proc Natl Acad Sci U S A 91(10):4130–4134
Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN (1998) Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G(1) arrest but not cell replication. Biochem J 332:43–50
Debacq-Chainiaux F, Borlon C, Pascal T, Royer V, Eliaers F, Ninane N, Carrard G, Friguet B, de Longueville F, Boffe S, Remacle J, Toussaint O (2005) Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta 1 signaling pathway. J Cell Sci 118:743–758
Debacq-Chainiaux F, Pascal T, Boilan E, Bastin C, Bauwens E, Toussaint O (2008) Screening of senescence-associated genes with specific DNA array reveals the role of IGFBP-3 in premature senescence of human diploid fibroblasts. Free Radic Biol Med 44(10):1817–1832
Dimri GP, Lee XH, Basile G, Acosta M, Scott C, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereirasmith O, Peacocke M, Campisi J (1995) A biomarker that identifies senescent human-cells in culture and in aging skin in-vivo. Proc Natl Acad Sci U S A 92(20):9363–9367
Dumont P, Burton M, Chen QM, Gonos ES, Frippiat C, Mazarati JB, Eliaers F, Remacle J, Toussaint O (2000) Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med 28(3):361–373
Dumont P, Chainiaux F, Eliaers F, Petropoulou C, Remacle J, Koch-Brandt C, Gonos ES, Toussaint O (2002) Overexpression of apolipoprotein J in human fibroblasts protects against cytotoxicity and premature senescence induced by ethanol and tert-butylhydroperoxide. Cell Stress Chaperones 7(1):23–35
Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O (2001) Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem 276(4):2531–2537
Frippiat C, Dewelle J, Remacle J, Toussaint O (2002) Signal transduction in H2O2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic Biol Med 33(10):1334–1346
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189(1–2):147–163
Goldstein S (1990) Replicative senescence—the human fibroblast comes of age. Science 249(4973):1129–1133
Greenberg SB, Grove GL, Cristofalo VJ (1977) Cell-size in aging monolayer-cultures. Vitro 13(5):297–300
Gupta A, Lutsenko S (2009) Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med Chem 1(6):1125–1142
Hayflick L (1965) Limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37(3):614–636
Hayflick L, Moorhead PS (1961) Serial cultivation of human diploid cell strains. Exp Cell Res 25(3):585–621
Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwal A (2000) Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-beta in human renal epithelial cells. J Biol Chem 275(52):40904–40909
Hwang ES, Yoon G, Kang HT (2009) A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci 66(15):2503–2524
Kumazaki T, Kobayashi M, Mitsui Y (1993) Enhanced expression of fibronectin during invivo cellular aging of human vascular endothelial-cells and skin fibroblasts. Exp Cell Res 205(2):396–402
LeckaCzernik B, Moerman EJ, Jones RA, Goldstein S (1996) Identification of gene sequences overexpressed in senescent and Werner syndrome human fibroblasts. Exp Gerontol 31(1–2):159–174
Martin JL, Baxter RC (1991) Transforming growth-factor-beta stimulates production of insulin-like growth factor-binding protein-3 by human skin fibroblasts. Endocrinology 128(3):1425–1433
Martin M, Elnabout R, Lafuma C, Crechet F, Remy J (1990) Fibronectin and collagen gene-expression during invitro aging of pig skin fibroblasts. Exp Cell Res 191(1):8–13
Materia S, Cater MA, Klomp LW, Mercer JF, La Fontaine S (2011) Clusterin (apolipoprotein J), a molecular chaperone that facilitates degradation of the copper-ATPases ATP7A and ATP7B. J Biol Chem 286(12):10073–10083
Ning W, Song RP, Li CJ, Park E, Mohsenin A, Choi AMK, Choi ME (2002) TGF-beta(1) stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 283(5):L1094–L1102
Pascal T, Debacq-Chainiaux F, Chretien A, Bastin C, Dabee AF, Bertholet V, Remacle J, Toussaint O (2005) Comparison of replicative senescence and stress-induced premature senescence combining differential display and low-density DNA arrays. FEBS Lett 579(17):3651–3659
Pascal T, Debacq-Chainiaux F, Boilan E, Ninane N, Raes M, Toussaint O (2007) Heme oxygenase-1 and interleukin-11 are overexpressed in stress-induced premature senescence of human WI-38 fibroblasts induced by tert-butylhydroperoxide and ethanol. Biogerontology 8(4):409–422
Poon S, Treweek TM, Wilson MR, Easterbrook-Smith SB, Carver JA (2002) Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett 513(2–3):259–266
Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3(7):1125–1131
Scheckhuber CQ, Grief J, Boilan E, Luce K, Debacq-Chainiaux F, Rittmeyer C, Gredilla R, Kolbesen BO, Toussaint O, Osiewacz HD (2009) Age-related cellular copper dynamics in the fungal ageing model Podospora anserina and in ageing human fibroblasts. PLoS One 4(3)
Smith JR, Pereira Smith OM (1996) Replicative senescence: implications for in vivo aging and tumor suppression. Science 273(5271):63–67
Soares MP, Bach FH (2009) Heme oxygenase-1: from biology to therapeutic potential. Trends Mol Med 15(2):50–58
Stein GH, Beeson M, Gordon L (1990) Failure to phosphorylate the retinoblastoma gene-product in senescent human fibroblasts. Science 249(4969):666–669
Toussaint O, Medrano EE, von Zglinicki T (2000) Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol 35(8):927–945
Trougakos IP, Gonos ES (2006) Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res 40(12):1324–1334
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1(3):1112–1116
von Zglinicki T, Saretzki G, Docke W, Lotze C (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts—a model for senescence. Exp Cell Res 220(1):186–193
Wang Y, Meng AM, Zhou DH (2004) Inhibition of phosphatidylinostol 3-kinase uncouples H2O2-induced senescent phenotype and cell cycle arrest in normal human diploid fibroblasts. Exp Cell Res 298(1):188–196
Zdanov S, Debacq-Chainiaux F, Remacle J, Toussaint O (2006) Identification of p38(MAPK)-dependent genes with changed transcript abundance in H2O2-induced premature senescence of IMR-90 hTERT human fibroblasts. FEBS Lett 580(27):6455–6463
Zhou B, Gitschier J (1997) hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci U S A 94(14):7481–7486
Acknowledgments
L. Matos would like to acknowledge “Fundação para a Ciência e Tecnologia” for her PhD grant [SFRH/BD/61820/2009].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource 1
Effect of 500 μM copper sulfate on mRNA transcript levels of several senescence-associated genes. The incubation of WI-38 fibroblasts with the lower cytotoxic copper concentration (500 μM) resulted in non-significant variations on p21, ApoJ, TGF β1, fibronectin, and HO-1 gene expression, when compared with controls. In addition, IGFBP3 mRNA levels were 1.7-fold increased in 500 μM CuSO4-treated cells, comparing with control cells. When compared with the consistent upregulation of the several senescence-associated genes observed for cells exposed to 250 μM copper, gene expression variations obtained with 500 μM copper sulfate were senseless, suggesting that the transcriptional machinery of these cells were seriously compromised due to the toxic effects of the dose. Data are presented as mean ± SEM from at least three independent experiments. *p < 0.05 when compared with control (JPEG 14 kb)
Online resource 2
Western blot analysis of fibronectin, p21, ApoJ, and TGF β1 protein levels in cells submitted to 500 μM copper sulfate. a Representative blots for the detection of the different proteins. Tubulin was used as loading control. b The resulting bands were quantified using densitometric analysis of the different signals. Protein levels of p21 were found significantly increased (3.7-fold) in WI-38 fibroblasts treated with 500 μM copper sulfate for 24h, when compared with controls. Both fibronectin and TGF β1 intracellular protein levels were decreased, and ApoJ protein content did not alter after exposure to the cytotoxic dose of copper, when compared with control cells. These inconsistent protein variations may originate from cellular metabolic alterations reflecting the cytotoxic effects of copper concentration used. Data are expressed as mean ± SEM from at least three independent experiments. *p < 0.05; **p < 0.01 when compared with control (TIFF 9255 kb) (JPEG 22 kb)
About this article
Cite this article
Matos, L., Gouveia, A. & Almeida, H. Copper ability to induce premature senescence in human fibroblasts. AGE 34, 783–794 (2012). https://doi.org/10.1007/s11357-011-9276-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-011-9276-7