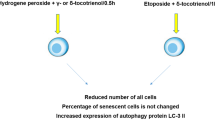
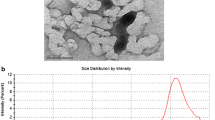
Abstract
Oxidative stress induces cell senescence and aging. Cell senescence is an irreversible cell cycle arrest mechanism responsible for various pathological diseases and aging. Cerium oxide nanoparticles (CeO2 NPs) have been found to have anti-oxidant effects with a capacity to scavenge free radicals. With this background, we aimed to investigate the senolytic property of CeO2 NPs on mouse embryonic fibroblasts (NIH3T3). NIH3T3 cells were exposed to CeO2 NPs with or without hydrogen peroxide (H2O2). The senolytic effect of CeO2 NPs was evaluated by measuring reactive oxygen species (ROS) production, lipid peroxidation (LPO), β-galactosidase, cell cycle phase analysis, and expression of p21, p38, p53, and NF-кB genes. EC50 of CeO2 NPs significantly reduced the β-galactosidase activity in NIH3T3 cells without apparent cytotoxicity and generation of ROS. Also, it decreased LPO, and expression of IL-6. The number of cells increased significantly, indicating that CeO2 NPs can reverse cell senescence and cell cycle arrest in the S phase. Also, p21, p38, p53, and NF-кB gene expressions were significantly down-regulated. CeO2 NPs as a senolytic or inhibitor of senescence can attenuate the senescence-inducing signal transduction pathways, i.e., p38/ NF-кB and p53/p21. It reduced the production of ROS and LPO and the expression of IL-6.
Graphic Abstract






Similar content being viewed by others
References
S. Khosla, J. N. Farr, T. Tchkonia, and J. L. Kirkland (2020). The role of cellular senescence in ageing and endocrine disease. Nature Reviews Endocrinology. 16 (5), 263–275.
J. M. van Deursen (2014). The role of senescent cells in ageing. Nature. 509 (7501), 439–446.
B. Bernardes de Jesus and M. A. Blasco (2012). Assessing cell and organ senescence biomarkers. Circ Res. 111 (1), 97–109.
R. Yosef, N. Pilpel, N. Papismadov, H. Gal, Y. Ovadya, E. Vadai, et al. (2017). p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. EMBO J. 36 (15), 2280–2295.
A. Rufini, P. Tucci, I. Celardo, and G. Melino (2013). Senescence and aging: the critical roles of p53. Oncogene. 32 (43), 5129–5143.
M. Mijit, V. Caracciolo, A. Melillo, F. Amicarelli, and A. Giordano (2020). Role of p53 in the regulation of cellular senescence. Biomolecules. 10 (3), 420.
Y. Qian and X. Chen (2013). Senescence regulation by the p53 protein family. Methods Mol Biol. 965, 37–61.
D. N. Krementsov, T. M. Thornton, C. Teuscher, and M. Rincon (2013). The emerging role of p38 mitogen-activated protein kinase in multiple sclerosis and its models. Mol Cell Biol. 33 (19), 3728–3734.
T. Davis, A. J. C. Brook, M. J. Rokicki, M. C. Bagley, and D. Kipling (2016). Evaluating the role of p38 MAPK in the accelerated cell senescence of Werner syndrome fibroblasts. Pharmaceuticals (Basel). 9 (2), 23.
J. Han and P. Sun (2007). The pathways to tumor suppression via route p38. Trends in Biochemical Sciences. 32 (8), 364–371.
A. Cuadrado and A. R. Nebreda (2010). Mechanisms and functions of p38 MAPK signalling. The Biochemical Journal. 429 (3), 403–417.
X. Chen, W. Zhang, Y. F. Gao, X. Q. Su, and Z. H. Zhai (2002). Senescence-like changes induced by expression of p21Waf1/Cip1 in NIH3T3 cell line. Cell Research. 12 (3), 229–233.
J. S. Tilstra, A. R. Robinson, J. Wang, S. Q. Gregg, C. L. Clauson, D. P. Reay, et al. (2012). NF-κB inhibition delays DNA damage–induced senescence and aging in mice. The Journal of Clinical Investigation. 122 (7), 2601–2612.
S. Vaughan and P. S. Jat (2011). Deciphering the role of nuclear factor-κB in cellular senescence. Aging (Albany NY). 3 (10), 913–919.
A. Rolt, A. Nair, and L. S. Cox (2019). Optimisation of a screening platform for determining IL-6 inflammatory signalling in the senescence-associated secretory phenotype (SASP). Biogerontology. 20 (3), 359–371.
H. Kojima, T. Inoue, H. Kunimoto, and K. Nakajima (2013). IL-6-STAT3 signaling and premature senescence. JAKSTAT. 2 (4), e25763.
C. R. Gomez, J. Karavitis, J. L. Palmer, D. E. Faunce, L. Ramirez, V. Nomellini, et al. (2010). Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators of Inflammation. 2010, 475139.
I. M. Rea, D. S. Gibson, V. McGilligan, S. E. McNerlan, H. D. Alexander, and O. A. Ross (2018). Age and Age-related diseases: role of inflammation triggers and cytokines. Frontiers in Immunology. 9, 586.
W. Li, L. Qin, R. Feng, G. Hu, H. Sun, Y. He, et al. (2019). Emerging senolytic agents derived from natural products. Mechanisms of Ageing and Development. 181, 1–6.
K. L. Heckman, A. Y. Estevez, W. DeCoteau, S. Vangellow, S. Ribeiro, J. Chiarenzelli, et al. (2020). Variable in vivo and in vitro biological effects of cerium oxide nanoparticle formulations. Frontiers in Pharmacology. 10, 1599.
Y. Li, X. Hou, C. Yang, Y. Pang, X. Li, G. Jiang, et al. (2019). Photoprotection of cerium oxide nanoparticles against UVA radiation-induced senescence of human skin fibroblasts due to their antioxidant properties. Scientific Reports. 9 (1), 2595.
G. Casals, M. Perramón, E. Casals, I. Portolés, G. Fernández-Varo, M. Morales-Ruiz, et al. (2021). Cerium oxide nanoparticles: a new therapeutic tool in liver diseases. Antioxidants (Basel). 10 (5), 660.
S. Mittal and A. K. Pandey (2014). Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. Biomed Res Int. 2014, 891934.
M. Rahimifard, M. Baeeri, H. Bahadar, S. Moini-Nodeh, M. Khalid, H. Haghi-Aminjan, et al. (2020). Therapeutic effects of gallic acid in regulating senescence and diabetes; an in vitro study. Molecules (Basel, Switzerland). 25, 5875.
M. M. Bradford (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72, 248–254.
S. M. Nejad, M. Hodjat, S. A. Mousavi, M. Baeeri, M. A. Rezvanfar, M. Rahimifard, et al. (2020). Alteration of gene expression profile in mouse embryonic stem cells and neural differentiation deficits by ethephon. Human & Experimental Toxicology. 39 (11), 1518–1527.
S. Das, J. Das, A. Samadder, N. Boujedaini, and A. R. Khuda-Bukhsh (2012). Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Experimental Biology and Medicine (Maywood, NJ). 237 (12), 1433–1448.
N. Sadeghiyan Galeshkalami, M. Abdollahi, R. Najafi, M. Baeeri, A. Jamshidzade, R. Falak, et al. (2019). Alpha-lipoic acid and coenzyme Q10 combination ameliorates experimental diabetic neuropathy by modulating oxidative stress and apoptosis. Life Sciences. 216, 101–110.
M. Baeeri, M. Rahimifard, S. M. Daghighi, F. Khan, S. A. Salami, S. Moini-Nodeh, et al. (2020). Cannabinoids as anti-ROS in aged pancreatic islet cells. Life Sciences. 256, 117969.
B. A. Rzigalinski, C. S. Carfagna, and M. Ehrich (2017). Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdisciplinary Reviews Nanomedicine and Nanobiotechnology. 9, 4.
S. Z. Nassar, P. S. Hassaan, D. A. Abdelmonsif, and S. N. ElAchy (2018). Cardioprotective effect of cerium oxide nanoparticles in monocrotaline rat model of pulmonary hypertension: a possible implication of endothelin-1. Life Sciences. 201, 89–101.
D. Oró, T. Yudina, G. Fernández-Varo, E. Casals, V. Reichenbach, G. Casals, et al. (2016). Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. Journal of Hepatology. 64 (3), 691–698.
S. Naz, J. Beach, B. Heckert, T. Tummala, O. Pashchenko, T. Banerjee, et al. (2017). Cerium oxide nanoparticles: a ‘radical’ approach to neurodegenerative disease treatment. Nanomedicine (London, England). 12 (5), 545–553.
D. Zhou, T. Fang, L. Q. Lu, and L. Yi (2016). Neuroprotective potential of cerium oxide nanoparticles for focal cerebral ischemic stroke. Journal of Huazhong University of Science and Technology Medical Sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 36 (4), 480–486.
M. Najafi, K. Mortezaee, M. Rahimifard, B. Farhood, and H. Haghi-Aminjan (2020). The role of curcumin/curcuminoids during gastric cancer chemotherapy: a systematic review of non-clinical study. Life Sciences. 257, 118051.
M. Samadi, H. Haghi-Aminjan, M. Sattari, M. R. Hooshangi Shayesteh, B. Bameri, M. Armandeh, et al. (2021). The role of taurine on chemotherapy-induced cardiotoxicity: a systematic review of non-clinical study. Life Sciences. 265, 118813.
N. Nobakht-Haghighi, M. Rahimifard, M. Baeeri, M. A. Rezvanfar, S. Moini Nodeh, H. Haghi-Aminjan, et al. (2018). Regulation of aging and oxidative stress pathways in aged pancreatic islets using alpha-lipoic acid. Molecular and Cellular Biochemistry. 449 (1–2), 267–276.
M. Armandeh, B. Bameri, M. Baeeri, H. Haghi-Aminjan, M. Rahimifard, S. Hassani, et al. (2021). The role of levosimendan in phosphine-induced cardiotoxicity: evaluation of electrocardiographic, echocardiographic, and biochemical parameters. Toxicology Mechanisms and Methods. https://doi.org/10.1080/15376516.2021.1950248.
H. Haghi-Aminjan, M. Baeeri, M. Rahimifard, A. Alizadeh, M. Hodjat, S. Hassani, et al. (2018). The role of minocycline in alleviating aluminum phosphide-induced cardiac hemodynamic and renal toxicity. Environmental Toxicology and Pharmacology. 64, 26–40.
K. Saravanakumar, A. Sathiyaseelan, A. V. A. Mariadoss, and M.-H. Wang (2021). Antioxidant and antidiabetic properties of biocompatible ceria oxide (CeO2) nanoparticles in mouse fibroblast NIH3T3 and insulin resistant HepG2 cells. Ceramics International. 47 (6), 8618–8626.
M. Alaraby, A. Hernández, B. Annangi, E. Demir, J. Bach, L. Rubio, et al. (2015). Antioxidant and antigenotoxic properties of CeO2 NPs and cerium sulphate: studies with Drosophila melanogaster as a promising in vivo model. Nanotoxicology. 9 (6), 749–759.
L. T. N. Ngoc, V. K. H. Bui, J. Y. Moon, and Y. C. Lee (2019). In-vitro cytotoxicity and oxidative stress induced by cerium aminoclay and cerium oxide nanoparticles in human skin keratinocyte cells. Journal of Nanoscience and Nanotechnology. 19 (10), 6369–6375.
R. Singh, A. S. Karakoti, W. Self, S. Seal, and S. Singh (2016). Redox-sensitive cerium oxide nanoparticles protect human keratinocytes from oxidative stress induced by glutathione depletion. Langmuir: The ACS Journal of Surfaces and Colloids. 32 (46), 12202–12211.
H. Ghaznavi, R. Najafi, S. Mehrzadi, A. Hosseini, N. Tekyemaroof, A. Shakeri-Zadeh, et al. (2015). Neuro-protective effects of cerium and yttrium oxide nanoparticles on high glucose-induced oxidative stress and apoptosis in undifferentiated PC12 cells. Neurological Research. 37 (7), 624–632.
A. Azari, M. Shokrzadeh, E. Zamani, N. Amani, and F. Shaki (2019). Cerium oxide nanoparticles protects against acrylamide induced toxicity in HepG2 cells through modulation of oxidative stress. Drug and Chemical Toxicology. 42 (1), 54–59.
K. Li, Y. Xie, M. You, L. Huang, and X. Zheng (2016). Plasma sprayed cerium oxide coating inhibits H2O2-induced oxidative stress and supports cell viability. Journal of Materials Science Materials in Medicine. 27 (6), 100.
O. A. Adebayo, O. Akinloye, and O. A. Adaramoye (2020). Cerium oxide nanoparticles attenuate oxidative stress and inflammation in the liver of diethylnitrosamine-treated mice. Biological Trace Element Research. 193 (1), 214–225.
M. Sheikhalipour, B. Esmaielpour, G. Gohari, M. Haghighi, H. Jafari, H. Farhadi, et al. (2021). Salt stress mitigation via the foliar application of chitosan-functionalized selenium and anatase titanium dioxide nanoparticles in stevia (Stevia rebaudiana Bertoni). Molecules (Basel, Switzerland). 26 (13), 4090.
S. Momtaz, M. Baeeri, M. Rahimifard, H. Haghi-Aminjan, S. Hassani, and M. Abdollahi (2019). Manipulation of molecular pathways and senescence hallmarks by natural compounds in fibroblast cells. Journal of Cellular Biochemistry. 120 (4), 6209–6222.
R. Di Micco, V. Krizhanovsky, D. Baker, and F. d’Adda di Fagagna (2021). Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology. 22 (2), 75–95.
R. G. Faragher, A. McArdle, A. Willows, and E. L. Ostler (2017). Senescence in the aging process. F1000Research. 6, 1219.
B. B. McConnell, M. Starborg, S. Brookes, and G. Peters (1998). Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Current Biology. 8 (6), 351–354.
D. Wu and C. Prives (2018). Relevance of the p53-MDM2 axis to aging. Cell Death and Differentiation. 25 (1), 169–179.
Y. Haupt, A. I. Robles, C. Prives, and V. Rotter (2002). Deconstruction of p53 functions and regulation. Oncogene. 21 (54), 8223–8231.
F. Lanigan, J. G. Geraghty, and A. P. Bracken (2011). Transcriptional regulation of cellular senescence. Oncogene. 30 (26), 2901–2911.
B.-D. Chang, K. Watanabe, E. V. Broude, J. Fang, J. C. Poole, T. V. Kalinichenko, et al. (2000). Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proceedings of the National Academy of Sciences. 97 (8), 4291–4296.
U. Herbig, W. Wei, A. Dutriaux, W. A. Jobling, and J. M. Sedivy (2003). Real-time imaging of transcriptional activation in live cells reveals rapid up-regulation of the cyclin-dependent kinase inhibitor gene CDKN1A in replicative cellular senescence. Aging Cell. 2 (6), 295–304.
J. Brugarolas, C. Chandrasekaran, J. I. Gordon, D. Beach, T. Jacks, and G. J. Hannon (1995). Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 377 (6549), 552–557.
X. D. Wang, E. Lapi, A. Sullivan, I. Ratnayaka, R. Goldin, R. Hay, et al. (2011). SUMO-modified nuclear cyclin D1 bypasses Ras-induced senescence. Cell Death & Differentiation. 18 (2), 304–314.
V. Dulić, L. F. Drullinger, E. Lees, S. I. Reed, and G. H. Stein (1993). Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proceedings of the National Academy of Sciences of the United States of America. 90 (23), 11034–11038.
V. Dulić, L. F. Drullinger, E. Lees, S. I. Reed, and G. H. Stein (1993). Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proceedings of the National Academy of Sciences. 90 (23), 11034–11038.
M. Mozaffar, M. A. Shahrbaf, B. Azimi, and A. Arabzadeh (2019). Management of celiac trunk and superior mesenteric artery synchronous aneurysms as an extremely rare manifestation of Wegener granulomatosis. Journal of Vascular Surgery Cases and Innovative Techniques. 5 (4), 525–528.
M. Hodjat, M. Baeeri, M. A. Rezvanfar, M. Rahimifard, M. Gholami, and M. Abdollahi (2017). On the mechanism of genotoxicity of ethephon on embryonic fibroblast cells. Toxicology Mechanisms and Methods. 27 (3), 173–180.
D. Laveti, M. Kumar, R. Hemalatha, R. Sistla, V. G. Naidu, V. Talla, et al. (2013). Anti-inflammatory treatments for chronic diseases: a review. Inflammation & Allergy Drug Targets. 12 (5), 349–361.
J. L. Ren, J. S. Pan, Y. P. Lu, P. Sun, and J. Han (2009). Inflammatory signaling and cellular senescence. Cellular Signalling. 21 (3), 378–383.
T. Zarubin and J. Han (2005). Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15 (1), 11–18.
H. Iwasa, J. Han, and F. Ishikawa (2003). Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes to Cells: Devoted to Molecular & Cellular Mechanisms. 8 (2), 131–144.
M. A. Stevenson, S. S. Pollock, C. N. Coleman, and S. K. Calderwood (1994). X-irradiation, phorbol esters, and H2O2 stimulate mitogen-activated protein kinase activity in NIH-3T3 cells through the formation of reactive oxygen intermediates. Cancer Research. 54 (1), 12–15.
J. Papaconstantinou (2019). The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells. 8 (11), 1383.
E. Rovillain, L. Mansfield, C. Caetano, M. Alvarez-Fernandez, O. L. Caballero, R. H. Medema, et al. (2011). Activation of nuclear factor-kappa B signalling promotes cellular senescence. Oncogene. 30 (20), 2356–2366.
A. Salminen, A. Kauppinen, and K. Kaarniranta (2012). Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cellular Signalling. 24 (4), 835–845.
R. NilamberLal Das, S. Muruhan, R. P. Nagarajan, and A. Balupillai (2019). Naringin prevents ultraviolet-B radiation-induced oxidative damage and inflammation through activation of peroxisome proliferator-activated receptor γ in mouse embryonic fibroblast (NIH-3T3) cells. Journal of Biochemical and Molecular Toxicology. 33 (3), e22263.
Acknowledgements
Research reported in this publication was supported by Elite Researcher Grant Committee under Award Number 977125 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran. Authors wish to thank Iran National Science Foundation (INSF) for general seat award support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All experiments were conducted per permission from the National Institute for Medical Research Development (NIMAD), Tehran, Iran, and were done according to the ethical approval number: IR.NIMAD.REC.1397.427.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghi-Aminjan, H., Baeeri, M., Khalid, M. et al. Senolytic Effect of Cerium Oxide Nanoparticles (CeO2 NPs) by Attenuating p38/NF-кB, and p53/p21 Signaling Pathways. J Clust Sci 33, 2265–2275 (2022). https://doi.org/10.1007/s10876-021-02152-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02152-y