Abstract
Lead (Pb) is a toxic metal originating from natural processes and anthropogenic activities such as coal power plants, mining, waste gas fuel, leather whipping, paint, and battery factories, which has adverse effects on the ecosystem and the health of human beings. Hence, the studies about investigating the remediation of Pb pollution have aroused extensive attention. Microbial remediation has the advantages of lower cost, higher efficiency, and less impact on the environment. This paper represented a review on the mechanism of biomineralization using microbial-induced precipitation for immobilizing Pb(II), including microbial-induced carbonate precipitation (MICP), microbial-induced phosphate precipitation (MIPP), and direct mineralization. The main mechanisms including biosorption, bioaccumulation, complexation, and biomineralization could decrease Pb(II) concentrations and convert exchangeable state into less toxic residual state. We also discuss the factors that govern methods for the bioremediation of Pb such as microbe characteristics, pH, temperature, and humic substances. Based on the above reviews, we provide a scientific basis for the remediation performance of microbial-induced precipitation technique and theoretical guidance for the application of Pb(II) remediation in soils and wastewater.
Similar content being viewed by others
Introduction
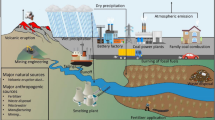
Lead (Pb) is a non-essential and excessively noxious metallic element which is widely distributed in nature (Sari et al. 2007; Wang and Chen 2006). Both lead and soluble lead salt have adverse effects to the health of the ecosystem and human beings (Anayurt et al. 2009; Subbaiah et al. 2011). They could rapidly accumulate in the human body through the food chains and respiration, resulting in remarkable damaging of the liver, intestines, and nervous system if the uptake of lead is excessive (Bai et al. 2014; Beier et al. 2013; Yang et al. 2013). With the increase of mining and industrial activities in recent decades, lead has become one of the most common hazardous heavy metal pollutions which exists extensively in the atmospheric, terrestrial, and marine environments (Binczycki et al. 2020; Sheng et al. 2004; Sun et al. 2015). According to the investigation by Duan et al. (2016), nearly 20% of sites in China are contaminated by Pb, whose concentration is higher than the regulatory limit value (80 mg kg−1). The Pb level of 30,430 mg kg−1 was even detected close to a lead and zinc melting plant in Fujian Province, China.
The origin of lead pollution on a global scale is divided into natural and anthropogenic sources (Fig. 1). The natural source of Pb is mainly derived from natural activities such as volcanic eruption dust, forest fire fumes, and sea salt aerosols. Anthropogenic sources of coal power plants, mining, waste gas fuel, leather whipping, paint, and battery factory account for the main compartment by the origin of Pb pollution during the past century (Kushwaha et al. 2018; Yousaf et al. 2018). As compared to other heavy metals, lead has a long biological half-time, which is relatively rough to degrade. Many studies on the lead emission species are being conducted, both on local and global scales (Sakata et al. 2014; Zhang et al. 2011). Temporal evolution of lead environmental emissions from 1930 to 2010 ranged from 0.8 to 3.6 Mt yr−1, which accounted to 173.8 Mt (Liang and Mao 2015). Meanwhile, the mining wastes and Pb production increase continuously and it has higher pollution level of Pb in various environmental compartments (Elom et al. 2014; Turner and Lewis 2018). In industrial areas, total Pb concentration can reach up to 10,000 mg kg−1, which is 100 to 1000 times higher than soil (Schwab et al. 2005). In aquatic ecosystems, Pb is often found in effluents discharged from battery production industries and it tends to settle at the bottom of the water where it accumulates in the tissues of aquatic biota (Kundu et al. 2016; Lombardi et al. 2010). In the process, Pb has increased significantly, which led to biomagnifications at different levels in the food chain and enter the human body, which would, in turn, cause harm to health. Therefore, it is vital to pour attention into controlling the Pb pollution. The biogeochemical behavior of Pb has a noxious impact on the biological system in the atmosphere-soil-water environment to a great extent by dissolution, exchange, reduction, oxidation, and residual speciation (Bridgestock et al. 2016; Fang et al. 2013; Sammut et al. 2010; Shahid et al. 2012). In general, the mobility and bioavailability decrease in the following order: exchange > carbonate > reduction > oxidation > residue. Compared to the first three speciation of Pb, residual speciation is more unreactive in nature conditions and it is arduous to be affected by environmental changes (Fuentes et al. 2008). Similarly, it can minimize the Pb geochemical cycle and reduce Pb plant uptake in the soil.
In general, the remediation methods for heavy metal pollution are mainly divided into physical, chemical, and biological remediation (Dhankhar and Hooda 2011; Rahman and Singh 2020; van Wyk 2011). Bioremediation is a process used to treat contaminated media, including water, soil, and subsurface material, by altering environmental conditions to stimulate the growth of microorganisms and degrade the target pollutants. The main mechanisms include biosorption, bioaccumulation, complexation, and biomineralization by microbes, which decrease Pb(II) concentrations and convert the exchangeable state into a residual state lowering its toxicity (Fig. 2). In terms of remediation technology, microbial remediation has the advantages of lower cost, higher efficiency, and less impact on the environment in comparison to the other two technologies in many cases (Mitra et al. 2018; Yari et al. 2015). Extensive studies have determined that the interaction between microorganism and heavy metal could mediate mineral formation either directly or indirectly, changing the physical and chemical properties of heavy metals and their compounds (Ojuederie and Babalola 2017; Tang et al. 2019). Furthermore, with the development of modern microbial technology, the domesticated microorganisms have better specificity and higher efficiency in removing heavy metals, which manifests a broader development prospect for the application of microorganisms in environmental pollution treatment and remediation (Bai et al. 2018; Kim et al. 2016; Zhang et al. 2016).
Lead immobilization mechanisms by microbes (adapted from figures in Bolan et al. (2020))
Microorganisms are widely distributed in ecosystems as the “Basic Regulator” of environmental chemistry. They could reduce the toxicity of lead by absorption, precipitation, oxidation, and reduction. Microbial activities also affect the process of plant root secretion by changing the pH and environmental constituent in order to convert lead into an insoluble state. Microbial mineralization is a common phenomenon in nature, and a variety of application objectives are achieved through the study of different mechanisms of microbial mineralization. Lead ions would react to form lead minerals with minimal solubility by the action of microbes, which reduce the leachability of lead (Macaskie et al. 2000; Wang and Chen 2010). Currently, some researchers have centered on the interaction between mineral and microbe, as well as the electron transferring of mineralizing process, which provide a description about the locations in the microbial cell of Pb2+ (Kushwaha et al. 2018; Pirbadian et al. 2014; Ren et al. 2018; Wu et al. 2015). However, with the diversity of microbial species and environmental deviation for Pb immobilization, the understanding about the adsorption and immobilization of lead by microorganisms in the process of metabolism is of great importance, so as to recognize the pollution sources of lead and improve bioremediation of lead pollution.
In recent years, many researches on biomineralization showed that extracellular polymeric substance (EPS) plays a crucial role in nanoparticle accumulation and binds/detoxifies heavy metals (Lyu et al. 2020; Teng et al. 2019; Wheaton et al. 2015; Zhang et al. 2020). Morillo Perez et al. (2008) reported that the combination capacity of EPS to Pb2+ was 303 mg g−1 in Paenibacillus jamilae. Rasulov et al. (2013) also found that Azotobacter chroococcum XZU1 could adsorb 22.38 mg g−1 of Pb through EPS.
Based on current research results, it can be concluded that microbes could induce Pb immobilization through various processes in a variety of microbial species and environmental conditions; the principal mechanisms include the precipitation of Pb–carbonates (microorganisms induced carbonate precipitation, MICP), the precipitation of Pb–phosphates (microbial-induced phosphate precipitation, MIPP), and the precipitation of Pb–microorganism (electron transferring between mineral and microbe). In view of the significance of inquiring the immobilization mechanism of Pb, we demonstrate a literature review which focuses on the feasible mechanisms and research progress in microorganism-induced Pb immobilization in mineralization.
The purpose of this part is to (i) provide a literature review on the experimental studies of microbial-induced precipitation process for Pb biomineralization in recent years, (ii) discuss microbe species and influencing factors of efficiency in biomineralization of lead ions, and (iii) highlight the future research considerations for growing microbial species with strong mineralization ability and improving the remediation performance of microbial-induced precipitation technique.
Mechanisms of biomineralization for immobilizing lead ions
Biomineralization of Pb ions via MICP
Microbial-induced carbonate precipitation (MICP) based on biomineralization is a novel method of soil remediation, which has been widely concerned by researchers. In the past few years, MICP has gradually become a prevailing approach for remediation of heavy metal-contaminated soil, which has attracted many researchers’ interest, and the detailed information was reflected in Table 1. MICP technique based on urease bacteria is widely used in ecological remediation due to its advantages of low energy consumption, species richness, and environmental friendliness. The capability of ureolytic bacteria to completely remove lead lies in its ability to efficiently hydrolyze urea to generate carbonate ions and elevate the pH to alkaline conditions (8.0–9.1), which promotes the precipitation of lead and calcium carbonate. Examples of “induced” mineralization are lead carbonate formation in Sporosarcina pasteurii, as the type of mineral produced depends largely on the surrounding environmental conditions.
MICP technology mainly includes urea hydrolysis, denitrification, trivalent iron reduction, and sulfate reduction (De Muynck et al. 2010). Among various MICP pathways, MICP using urea as the substrate is one of the most popular pathways, which is originated from a wide range of microbes in soil (Castanier et al. 1993; DeJong et al. 2006; Gollapudi et al. 1995). Many extracellular polymeric substances are produced during the microbial growth process, which have negatively charged functional groups (–COOH, –OH, and C=O), adsorbing heavy metal cations from the solution (Fig. 3a). Ca2+ and CO32− combine to form CaCO3 precipitate under strong alkaline conditions, while coprecipitating Pb ions. At last, it mediates Pb ion mineralization as a result of precipitating lead-containing carbonate minerals on cell surface as shown in Fig. 3b. The biochemical reaction equations involving urealysis-driven MICP are as follows (Stocks-Fischer et al. 1999):
The process of biomineralization of heavy metal ions through MICP (adapted from figures in Fujita et al. (2004) and Mitchell and Ferris (2005)). a Calcium carbonate deposits physically close the migration pathway of heavy metal; b heavy metal ions exchanged with Ca2+ in the process of microbial mineralization to form carbonate deposits. After a while, heavy metal ions replaced the original position of Ca2+ in the CaCO3 lattice, resulting in forming calcium salt-heavy metal composite precipitation
where Mx+ represents the metal cation ions, such as Ca2+ and Pb2+.
Based on the above reactions, microbial metabolism, extracellular molecules, and the cell wall of microbes play a crucial role in the biomineralization of MICP because various negatively charged functional groups (such as carbonyl, carboxyl, and hydroxyl groups) could bind to heavy metal ions in the cell wall of the microorganism and complete heterogeneous nucleation process.
In the MICP biomineralization process, besides providing crystal nucleus, metabolic activity like urease produced from microbe could break the covalent bond of urea and form urease-urea reaction intermediates through a short-range non-covalent bond (hydrogen bond, ionic bond, and hydrophobic bond) between the active center of urease and urea molecules (Lu et al. 2011). Achal et al. (2012) reported bioremediation of Pb-contaminated soils using the MICP technique for the first time, which showed that Kocuria flava possessed the high capability for Pb2+ immobilization in the form of minerals (lead carbonate, lead oxides, and hydrocerussite) in contaminated soil. Up to now, some researchers have successively carried out studies on the application of the MICP technique to the remediation of Pb pollution (Jiang et al. 2019; Kim and Lee 2019; Mwandira et al. 2019; Wang et al. 2015). For example, He et al. (2019) investigated the MICP potential of ureolytic bacteria (Staphylococcus epidermidis) for the remediation of divalent Pb in solution with initial 25 mg L−1 PbCl2 concentration, and the maximum removal efficiency of Pb(II) reached up to 86%; Staphylococcus epidermidis could transform Pb(II) into calcite crystals in this process. Gomaa (2019) and Wang et al. (2015) confirmed that the Pb ions could be coprecipitated with CaCO3 in bioremediation as PbCO3 by Micrococcus sp. and Pseudomonas stutzeri, respectively. Qian et al. (2017) focused on studying the ability of Penicillium chrysogenum CS1 to remove Pb ions via calcium carbonate precipitation induced; it turns out that the fungi were contributed to the typical form of carbonate crystals (calcite, vaterite, and hydrocerussite) containing Pb element on the mycelia as reflected by the scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDX), and Fourier transform infrared spectroscopy (FTIR). However, the MICP technology is still at the stage of small-scale and pilot-scale trials, mainly due to the following reasons that limit its large-scale application: (1) nutrients, (2) calcium source, (3) urea, and (4) long-term storage and transportation of microbes. Therefore, the biomineralization ability of Pb can be improved in the following methods under real-life conditions: (1) by-products from the food industry substitute for nutrients from standard medium, (2) seawater and calcareous sand substitutes for calcium salts, (3) CO2 and industrial by-product substitute for urea, and (4) stimulating the growth of urease-producing microbes in a natural environment to solve the problem of long-term storage and transport of microbes.
Biomineralization of Pb ions via MIPP
Lead phosphates, which can reduce the bioavailability of lead (Nriagu 1973), are formed in contaminated soils under a wide range of geochemical conditions (Cotterhowells 1994; Davis et al. 1993; Ruby et al. 1994). It was considered that adding of phosphate could promote the formation of lead phosphates in order to remediate lead-contaminated land in previous studies (CotterHowells and Caporn 1996; Ruby et al. 1994). The MIPP process is to increase metabolic activity like phosphatase or phytase secreted by microbes according to adding a phosphate source, thereby precipitating Pb ions and attaching them to the cell surface in the form of lead-containing phosphate minerals. With the continuous development of scientific research, the MIPP biomineralization technique exhibits a great potential to efficiently remediate Pb-polluted soil environment. Sayer et al. (1999) demonstrated that lead oxalate and lead oxalate dihydrate were produced by an organic-acid-producing fungus (Aspergillus niger) through microbial phosphate-solubilizing mechanisms during pyromorphite [Pb5(PO4)3Cl] transformation, which was the first time that the biogenesis of this mineral has been recorded. Since then, many researchers found that, under various environmental conditions, a number of isolated bacteria and fungi could immobilize Pb ions by converting inorganic phosphate sources (i.e., apatite minerals) or organic phosphate sources (i.e., phenolphthalein diphosphate, glycerophosphate, acephate, glycerol 2-phosphate, and phytic acid) into phosphate in the presence of phosphatase or phytase enzyme. Phosphate-dissolving bacteria (PSB) have been extensively studied for immobilizing Pb ions; it could release alkalinity substance, phosphatase, or phytase in the process of degradation of organic phosphorus (Darch et al. 2016; Tahir et al. 2018; Wei et al. 2018). And then, these substances by PSB metabolism could increase the solubilization capacity of insoluble phosphates, promoting the rapid formation of lead phosphate [Pb(PO4)X(X = F, Cl, Br, OH)] in lead-contaminated soil (Cao and Ma 2004; Park et al. 2011a, b; Zhu et al. 2019). Pb ions are immobilized on the cell surfaces by phosphatase/phytase and some anions (Fig. 4). The biochemical reaction equations involving MIPP are as follows:
where Xx- represents the halogen ions (such as F−, Cl−, and Br−) or hydroxide groups (OH−). At present, three potential mechanisms have been suggested to explain the bioprecipitation process of phosphates: (i) the metabolites secreted by microorganisms are involved in the biomineralization process of MIPP (Roeselers and Van Loosdrecht 2010); (ii) extracellular molecules interact with phosphate to complete the mineralization process (Zhang et al. 2019); (iii) phosphate minerals biomineralize with Pb ions in the cell wall (Xu et al. 2020). Owing to binding of various negatively charged groups to positively charged Pb ions on the surface of microbes (heterogeneous nucleation), cell surfaces play a crucial role in the MIPP process.
In recent years, a number of microbe species are known to precipitation Pb on the cell surface in the form of lead phosphate compounds, such as Citrobacter sp., Vibrio harveyi, and Pseudomonas fluorescens (Ala et al. 1991; Mire et al. 2004). The mechanism of MIPP and the utilization of Pb in immobilization have already made strides to handle Pb pollution in soil (Table 2). For example, Su et al. (2020) illustrated that the hydroxyl group, amide group, and phosphate group on Rhodobacter sphaeroides SC01 and Pb ions complexing reactions accounted for their precipitation of lead phosphate hydroxide on the cell surface. Park et al. (2011a, b) found Enterobacter cloacae could reduce the mobility of lead by releasing organic phosphatases to combine lead with rock phosphate to form minerals. Lin et al. (2016) investigated the removal of Pb2+ by Bacillus subtilis FZUL-33 in the mix acephate-Pb2+ system and discovered PO43− generated by the biodegradation of phosphorous could lead to the biomineralization of Pb2+ to form Pb5(PO4)3OH precipitation. Teng et al. (2019) reported that Pb2+ formed into Pb5(PO4)3OH and Pb5(PO4)3Cl crystals through phosphatase released by Leclercia adecarboxylata L1-5.
Different kinds of yeasts also could exhibit the ability that affects changes in Pb speciation. They could mediate precipitation of lead phosphate [Pb3(PO4)2], pyromorphite [Pb5(PO4)3Cl], anglesite (PbSO4), litharge (PbO), and other lead oxides, respectively, which was cultured in medium containing organic phosphorus sources (Liang et al. 2016). The mineral morphology of these precipitations on the yeast cell walls is controlled by a number of factors, including yeast species, pH value, and coexistence of other metal cations (Ayati and Madsen 2000; Gadd 2009; Ohnuki et al. 2005).
Immobilization of Pb directly induced by a microorganism
In recent years, a large number of indigenous bacteria and fungi have been found to be able to mineralize and consolidate heavy metal cations according to electron transferring between minerals and microbes (Table 3). Alishewanella sp. (Zhou et al. 2016), Aspergillus niger (Ding et al. 2019; Dursun et al. 2003; Wang et al. 2001), Penicillium sp. (Xu et al. 2020), Saccharum bengalense (Din et al. 2014), Lentinus edodes, Pleurotus eryngii, Flammulina velutipes, Hypsizygus marmoreus, and Agrocybe cylindracea (Jiang et al. 2016) are capable of bioremediation of Pb pollution. These isolated bacteria and fungi have the ability to immobilize through multiple pathways (see Section 1 for details). This part focuses on the direct induction of Pb ion mineralization by microorganisms, particularly on current knowledge of several lead mineralized products and strategies for Pb removal from the environment. Other kinds of precipitates may be generated to immobilize lead ions in the presence of microbes except for the formation of lead-containing phosphate and carbonate minerals. Wang et al. (2017) reported the effect of absorption and immobilization of Pb by Penicillium polonicum under different voltage conditions based on doubled chamber electrolytic cell system. It is observed that the rate of Pb(II) removal by the fungi was improved greatly under voltage between 1.0 V and 1.25 V, and some well-formed columnar single crystals were produced. Wu et al. (2015) analyzed the immobilization to Pb2+ of Aspergillus tubingensis LYF12 isolated from contaminated soils in Pb-Zn mining areas; the results found that this strain contained some Pb mineral aggregations within the cell and implied that active transport might be the main way to transport Pb2+ into the cell. Jiang et al. (2020) found that lead nano-particles (NPs) accumulated on vesicles of yeast cells in the presence of lead ions.
Oxalic acid is a metabolite, which can play an important role in the stress responses of microbes to toxic heavy metals (Jarosz-Wilkolazka and Gadd 2003). Therefore, many studies have shown that lead oxalate minerals were formed by microorganisms in the process of immobilization of Pb2+. For example, Tian et al. (2019) showed that there were three pathways to resist Pb toxicity by Aspergillus niger and Penicillium oxalicum, namely, secretion of oxalic acid reacted with Pb(II) to form Pb minerals (primarily lead oxalate), biosorption via forming new border of cell wall, and intracellular accumulation. Zeng et al. (2015) used the living cells of Phanerochaete chrysosporium to remove Pb(II) from the Pb-contaminated environment and determined that stable lead oxalate and lead chloride phosphate would be formed in this process. Xu et al. (2020) indicated that Penicillium polonicum could immobilize Pb(II) as lead oxalate (PbC2O4) and pyromorphite [Pb5(PO4)3Cl] outside the cell and reduce Pb(II) to Pb(0) inside the cell. Ding et al. (2019) studied bioremediation of Pb contamination through the synergistic effect of anatase and Aspergillus niger under visible light irradiation, as result of removing Pb 803.16 mg L−1 for 5-6 days and forming extracellular lead-containing product (mainly lead oxalate). The method enormously accelerated the removal rate of Pb by Aspergillus niger. Jalili et al. (2020) confirmed that Pb immobilization in the contaminated mine soil was caused by Aspergillus niger SANRU, and the lead oxalate was formed in the spiked soil. Li et al. (2016) have found that Pb2+ could be immobilized by the mixture Aspergillus niger and geological fluorapatite, which formed FAL (fluoropyromorphite) and lead oxalate.
In addition, some bacteria and fungi have been reported to synthesize PbS based on Pb ions (Gong et al. 2007; Kowshik et al. 2002; Priyanka et al. 2017; Seshadri et al. 2011). Zhang and Huang (2020) confirmed that Pb2+ was adsorbed on the Shinella zoogloeoides PQ7 cells and transformed into PbS nanoparticles. Zhou et al. (2016) injected Alishewanella sp.WH16-1 into the Pb-contaminated soil where rice was cultivated; the results showed that the strain in pot experiments of contaminated paddy soil could promote the binding of Pb(II) with Fe-Mn oxide and organic matter or form PbS crystal so as to reduce the bioavailability of Pb and improve the yield and quality of rice. Moreover, the combination of bacterium and fungus might strengthen the removal of Pb. Compared with single bacteria, Pb immobilization was improved when bacterial-fungal consortia in the presence of nematodes were cultured (Iqbal et al. 2020).
Influencing factors
Microbial characteristics, as well as environmental factors such as pH, temperature, and environmental conditions (coexisting ions and humic substances), are the main factors determining the mobility and bioavailability of Pb for biomineralization performance, since they can directly affect the available valence and speciation of substance in the environment and then change the occurrence form of heavy metal ions through the mineralization processes (Daryono et al. 2019; Duarte-Nass et al. 2020; Kang et al. 2014). Thus, the remediation performance of microbial-induced lead ion precipitation in pollution requires a comprehensive understanding of its influencing factors. A detailed understanding of these influencing factors with some recent examples of microbial-induced precipitation for immobilizing Pb is elucidated in the following part.
Microbial characteristics
The enzymatic activity of microorganisms can affect the microbial-induced precipitation process of Pb ions. Chen et al. (2016) have found out the impact of enzymes of Bacillus cereus 12-2 on Pb(II) immobilization; results exhibited that the biosorption and biomineralization (Pb transformed into Ca2.5Pb7.5(OH)2(PO4)6) capacity with heat and 0.5% sodium dodecyl sulfate treatment was differently decreased in comparison to control groups under the same conditions, because of the lack of enzymes in the cell. Besides, many researchers studied that the phosphatase activity was positively correlated with the ability to immobilize Pb ions, on account of the transformation of organophosphorus complexes to inorganic phosphate minerals (see Section 2.2 for details). Moreover, the structures of microbe cell also influence the biomineralization process of Pb(II). The functional groups on the cell of some microbes provided nucleation sites for Pb ion bioprecipitation. For example, Mota et al. (2016) found that the hydroxyl (O-H) and carboxyl (C-H) group on the cytoderm of Cyanothece sp. CCY 0110 arose stretching vibration in an aqueous solution containing Pb ions, leading to better immobilization performance of Pb(II) on the surface of the cytoderm.
Environmental factors
pH is of vital importance in the immobilization of lead by microbes in Pb-contaminated environment, which could affect microbial quantity and enzyme activity. For example, Lin et al. (2016) studied the impact of pH values of Bacillus subtilis FZUL-33 on Pb2+ immobilization; the bioprecipitation of Pb2+ occurred mostly when pH = 5.5. The morphology of the minerals precipitated on the microbial cell surface also could be influenced by pH value (Liang et al. 2016). Ayati and Madsen (2000) found that lead phosphate mainly consists of PbHPO4 at low pH. However, it will change to be mainly in the form of small Pb5OH(PO4)3 crystals with the increase of pH value. Besides, the amount of humic acid and fulvic acid secreted by microbes is also affected by pH value and influenced the formation of Pb minerals. Li et al. (2013) reported that the biomineralization process was very fast and crystal structure of product was well when pH was 8–9. Qian and Zhan (2016) found that too high or too low pH would inhibit the production of phosphate by phosphate-mineralizing bacteria, suggesting pH greatly influences the immobilization rate. Humic substances have a good strong complexation ability to heavy metal cations, leading to decreasing the migration of Pb cations. Many researchers have found that a significant negative correlation between pH of rock phosphate amended liquid medium and solubilized P (Bolan et al. 2003; El-Tarabily et al. 2008), which suggests that pH value could indirectly affect MIPP efficiency.
Temperature is an important factor influencing the growth rate and metabolic activity level of microorganisms. The activity of enzymes such as urease and phosphatase varies with temperature, resulting in the change of biomineralization ability through MICP and MIPP. For example, Xu et al. (2013) found that an increase of temperature from 15 to 30°C decreased the urea concentration, suggesting that the urease activity released by carbonate-mineralizing bacteria was correlated with the temperature. Qian and Zhan (2016) examined the growth rate of phosphate-mineralizing bacteria varied with temperature. When the temperature was 30°C, the strain grew rapidly and released much alkalinity substance and phosphatase. Meanwhile, in the appropriate range, the adsorption ability of a microorganism to Pb2+ increases with the rising of temperature, which decreases the migration of Pb (Bandowe et al. 2014).
Future prospects
The remediation of Pb contamination through microbial-induced precipitation is a novel strategy. The remediation extent and efficiency are controlled by some factors, including microbial species and necessary environmental conditions. Although many recent researches have focused on the feasibility and mechanism of microbial Pb removal in the environment, the present approach for microbial-induced precipitation still has great opportunities and challenges on the further research:
-
(1)
The species and physiological characteristics of microorganisms affect the performance of biomineralization, such as the pH and temperature, organic acid production, available enzyme activity, coexisting ions, and Pb ion tolerance. Hence, the isolation of strain which has high Pb resistance and cell viability is a major research direction in the future.
-
(2)
Studies on the remediation of Pb ions by microbial-induced precipitation are mostly focused on small-scale laboratory experiments, and there was currently a lack of research on microbial in situ mineralization remediation technology and the validation of long-term restoration effect. Therefore, it is necessary to continue to long-term and continuous monitoring from the aspects of lead speciation, microbial activity, and community, so as to deeply explore the coevolution mechanism between microbial community and environmental factors in the process of in situ microbial mineralization and pollution remediation.
Conclusion
This review shows processes for the formation of lead minerals during Pb immobilization and the potential of remediation of environment contaminated by heavy metal through microbial activities. Besides, various influencing factors affecting biomineralization are also summarized. The microbial communities can directly or indirectly mediate the formation of Pb-containing minerals, which lead to the immobilization of Pb in the environment. There is a great deal of diversities in the adsorption and immobilization mechanism of different kinds of Pb-resistant microorganisms and the status of Pb ions. In order to promote bioremediation efficiency of Pb2+, future research should focus on exploring high-efficient microbes and improving the activity of available microorganisms by optimization of environmental conditions. In addition, it is extremely crucial to continue to long-term and continuous monitoring from the aspects of lead speciation, microbial activity, and community in situ field, in order to achieve the successful remediation performance under real-life conditions.
Data and materials availability
Not applicable.
References
Achal V, Pan XL, Zhang DY, Fu QL (2012) Bioremediation of Pb-contaminated soil based on microbially induced calcite precipitation. J Microbiol Biotechnol 22:244–247. https://doi.org/10.4014/jmb.1108.08033
Ala AA, Appanna VD, John H (1991) Exocellular and intracellular accumulation of lead in Pseudomonas fluorescens ATCC 13525 is mediated by the phosphate content of the growth medium. FEMS Microbiol Lett 83(3):283–290. https://doi.org/10.1111/j.1574-6968.1991.tb04478.x
Anayurt RA, Sari A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem Eng J 151:255–261. https://doi.org/10.1016/j.cej.2009.03.002
Ayati M, Madsen HEL (2000) Crystallization of some heavy-metal phosphates alone and in the presence of calcium ion. J Cryst Growth 208:579–591. https://doi.org/10.1016/s0022-0248(99)00403-0
Bai J, Yang XH, Du RY, Chen YM, Wang SZ, Qiu RL (2014) Biosorption mechanisms involved in immobilization of soil Pb by Bacillus subtilis DBM in a multi-metal-contaminated soil. J Environ Sci 26:2056–2064. https://doi.org/10.1016/j.jes.2014.07.015
Bai NL, Wang S, Sun PF, Abuduaini R, Zhu XF, Zhao YH (2018) Degradation of nonylphenol polyethoxylates by functionalized Fe3O nanoparticle-immobilized Sphingomonas sp. Y2. Sci Total Environ 615:462–468. https://doi.org/10.1016/j.scitotenv.2017.09.290
Bandowe BAM, Bigalke M, Boamah L, Nyarko E, Saalia FK, Wilcke W (2014) Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): bioaccumulation and health risk assessment. Environ Int 65:135–146. https://doi.org/10.1016/j.envint.2013.12.018
Beier EE, Maher JR, Sheu TJ, Cory-Slechta DA, Berger AJ, Zuscik MJ, Puzas JE (2013) Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ Health Perspect 121:97–104. https://doi.org/10.1289/ehp.1205374
Binczycki T, Weber J, Mielnik L, Asensio C (2020) Lead isotope ratios in Podzol profiles as a tracer of pollution source in the subalpine zone of the Karkonosze National Park, Sudety Mts (south-western Poland). Catena 189:8. https://doi.org/10.1016/j.catena.2020.104476
Bolan NS, Adriano DC, Naidu R (2003) Role of phosphorus in (im)mobilization and bioavailability of heavy metals in the soil-plant system. Rev Environ Contam Toxicol 177:1–44. https://doi.org/10.1007/0-387-21725-8_1
Bolan S, Seshadri B, Grainge I et al (2020) Gut microbes modulate bioaccessibility of lead in soil. Chemosphere 12:128657
Bridgestock L, van de Flierdt T, Rehkämper M, Paul M, Middag R, Milne A, Lohan MC, Baker AR, Chance R, Khondoker R, Strekopytov S, Humphreys-Williams E, Achterberg EP, Rijkenberg MJA, Gerringa LJA, de Baar HJW (2016) Return of naturally sourced Pb to Atlantic surface waters. Nat Commun 7:12. https://doi.org/10.1038/ncomms12921
Cao XD, Ma LQ (2004) Effects of compost and phosphate on plant arsenic accumulation from soils near pressure-treated wood. Environ Pollut 132(3):435–442. https://doi.org/10.1016/j.envpol.2004.05.019
Castanier S, Bernetrollande MC, Maurin A, Perthuisot JP (1993) Effects of microbial activity on the hydrochemistry and sedimentology of Lake Logipi, Kenya. Hydrobiologia. 267:99–112. https://doi.org/10.1007/bf00018793
Chen Z, Pan XH, Chen H, Guan X, Lin Z (2016) Biomineralization of Pb(II) into Pb-hydroxyapatite induced by Bacillus cereus 12-2 isolated from Lead-Zinc mine tailings. J Hazard Mater 301:531–537. https://doi.org/10.1016/j.jhazmat.2015.09.023
Cheng Y, Zhao XQ (2016) Study on the consolidation and mineralization of Pb2+ by carbonate-mineralization bacteria. Res Environ Sci 29(10):1513–1520
Cotterhowells J (1994) Lead phosphate formation in soils. Environ Geochem Health 16:86–86. https://doi.org/10.1007/bf00209835
CotterHowells J, Caporn S (1996) Remediation of contaminated land by formation of heavy metal phosphates. Appl Geochem 11:335–342. https://doi.org/10.1016/0883-2927(95)00042-9
Darch T, Blackwell MSA, Chadwick D, Haygarth PM, Hawkins JMB, Turner BL (2016) Assessment of bioavailable organic phosphorus in tropical forest soils by organic acid extraction and phosphatase hydrolysis. Geoderma 284:93–102. https://doi.org/10.1016/j.geoderma.2016.08.018
Daryono LR, Nakashima K, Kawasaki S, Titisari AD, Barianto DH, Suyanto I, Rahmadi A (2019) Biomineralization of an artificial beachrock based on urease microbial activities for coastal risk prevention. ICBME2019 2155(1):020046. https://doi.org/10.1063/1.5125550
Davis A, Drexler JW, Ruby MV, Nicholson A (1993) Micromineralogy of mine wastes in relation to lead bioavailability, Butte, Montana. Environ Sci Technol 27:1415–1425. https://doi.org/10.1021/es00044a018
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36:118–136. https://doi.org/10.1016/j.ecoleng.2009.02.006
DeJong JT, Fritzges MB, Nusslein K (2006) Microbially induced cementation to control sand response to undrained shear. J Geotech Geoenviron Eng 132:1381–1392. https://doi.org/10.1061/(asce)1090-0241(2006)132:11(1381)
Dhankhar R, Hooda A (2011) Fungal biosorption – an alternative to meet the challenges of heavy metal pollution in aqueous solutons. Environ Technol 32(5-6):467–491. https://doi.org/10.1080/09593330.2011.572922
Din MI, Hussain Z, Mirza ML, Shah AT, Athar MM (2014) Adsorption optimization of lead (II) using Saccharum bengalense as a non-conventional low cost biosorbent: isotherm and thermodynamics modeling. Int J Phytoremediation 16:889–908. https://doi.org/10.1080/15226514.2013.803025
Ding Y, Hao RX, Xu XY, Lu AH, Xu H (2019) Improving immobilization of Pb(II) ions by Aspergillus niger cooperated with photoelectron by anatase under visible light irradiation. Geomicrobiol J 36:591–599. https://doi.org/10.1080/01490451.2019.1594464
Duan QN, Lee JC, Liu YS, Chen H, Hu HY (2016) Distribution of heavy metal pollution in surface soil samples in China: a graphical review. Bull Environ Contam Toxicol 97:303–309. https://doi.org/10.1007/s00128-016-1857-9
Duarte-Nass C, Rebolledo K, Valenzuela T, Kopp M, Jeison D, Rivas M, Azócar L, Torres-Aravena Á, Ciudad G (2020) Application of microbe-induced carbonate precipitation for copper removal from copper-enriched waters: challenges to future industrial application. J Environ Manag 256:10. https://doi.org/10.1016/j.jenvman.2019.109938
Dursun AY, Uslu G, Cuci Y, Aksu Z (2003) Bioaccumulation of copper(II), lead(II) and chromium(VI) by growing Aspergillus niger. Process Biochem 38:1647–1651. https://doi.org/10.1016/s0032-9592(02)00075-4
Elom NI, Entwistle J, Dean JR (2014) Human health risk from Pb in urban street dust in northern UK cities. Environ Chem Lett 12:209–218. https://doi.org/10.1007/s10311-013-0436-0
El-Tarabily KA, Nassar AH, Sivasithamparam K (2008) Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl Soil Ecol 39:161–171. https://doi.org/10.1016/j.apsoil.2007.12.005
Fang Y, Yang WJ, Chen Y, Ma N, Hu QH (2013) Chemical speciation of heavy metal lead and its food safety. J Chin Cereal Oil Ass 28(6):123–128. https://doi.org/10.3969/j.issn.1003-0174.2013.06.026
Fuentes A, Llorens M, Saez J, Aguilar MI, Ortuno JF, Meseguer VF (2008) Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour Technol 99:517–525. https://doi.org/10.1016/j.biortech.2007.01.025
Fujita Y, Redden GD, Ingram JC, Cortez MM, Ferris FG, Smith RW (2004) Strontium incorporation into calcite generated by bacterial ureolysis. Geochim Cosmochim Acta 68:3261–3270. https://doi.org/10.1016/j.gca.2003.12.018
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28. https://doi.org/10.1002/jctb.1999
Gollapudi UK, Knutson CL, Bang SS, Islam MR (1995) A new method for controlling leaching through permeable channels. Chemosphere 30:695–705. https://doi.org/10.1016/0045-6535(94)00435-w
Gomaa EZ (2019) Biosequestration of heavy metals by microbially induced calcite precipitation of ureolytic bacteria. Appl Microbiol BioT 24:147–153. https://doi.org/10.25083/rbl/24.1/147.153
Gong J, Zhang Z, Bai H, Yang Guan E (2007) Microbiological synthesis of nanophase PbS by Desulfotomaculum sp. Scientia Sinica(Technologica) 50:302–307. https://doi.org/10.1007/s11431-007-0045-x
He J, Chen XY, Zhang QZ, Achal V (2019) More effective immobilization of divalent lead than hexavalent chromium through carbonate mineralization by Staphylococcus epidermidis HJ2. Int Biodeterior Biodegrad 140:67–71. https://doi.org/10.1016/j.ibiod.2019.03.012
Iqbal N, Ahmed S, Pervez A, Nazir R, Tang XY, Irshad U (2020) The fate of lead (Pb) in multitrophic interactions among bacteria, fungi, and bacterivorous soil nematodes. Clean-Soil Air Water 48(11):2000307. https://doi.org/10.1002/clen.202000307
Jalili B, Sadegh-Zadeh F, Jabari-Giashi M, Emadi M (2020) Lead bioimmobilization in contaminated mine soil by Aspergillus niger SANRU. J Hazard Mater 393:7. https://doi.org/10.1016/j.jhazmat.2020.122375
Jalilvand N, Akhgar A, Alikhani HA, Rahmani HA, Rejali F (2020) Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian mine calcareous soils. J Soil Sci Plant Nutr 20:206–219. https://doi.org/10.1007/s42729-019-00121-z
Jarosz-Wilkolazka A, Gadd GM (2003) Oxalate production by wood-rotting fungi growing in toxic metal-amended medium. Chemosphere 52:541–547. https://doi.org/10.1016/s0045-6535(03)00235-2
Jiang Y, Hao R, Yang S (2016) Equilibrium and kinetic studies on biosorption of Pb(II) by common edible macrofungi: a comparative study. Can J Microbiol 62:329–337. https://doi.org/10.1139/cjm-2015-0631
Jiang NJ, Liu R, Du YJ, Bi YZ (2019) Microbial induced carbonate precipitation for immobilizing Pb contaminants: toxic effects on bacterial activity and immobilization efficiency. Sci Total Environ 672:722–731. https://doi.org/10.1016/j.scitotenv.2019.03.294
Jiang ZQ, Wang T, Sun Y, Nong Y, Tang L, Gu T, Wang S, Li Z (2020) Application of Pb(II) to probe the physiological responses of fungal intracellular vesicles. Ecotoxicol Environ Saf 194:8. https://doi.org/10.1016/j.ecoenv.2020.110441
Kang CH, Han SH, Shin Y, Oh SJ, So JS (2014) Bioremediation of Cd by microbially induced calcite precipitation. Appl Biochem Biotechnol 172:2907–2915. https://doi.org/10.1007/s12010-014-0737-1
Kang CH, Oh SJ, Shin YJ, Han SH, Nam IH, So JS (2015) Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol Eng 74:402–407. https://doi.org/10.1016/j.ecoleng.2014.10.009
Kim JH, Lee JY (2019) An optimum condition of MICP indigenous bacteria with contaminated wastes of heavy metal. J Mater Cycles Waste 21:239–247. https://doi.org/10.1007/s10163-018-0779-5
Kim IH, Lee WJ, Kim YK, Oh BK (2016) Development of recycled aggregate bio-carrier with sulfate reducing bacteria for the elimination of heavy metals from seawater. Biotechnol Bioprocess Eng 21:689–693. https://doi.org/10.1007/s12257-016-0474-0
Kowshik M, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2002) Microbial synthesis of semiconductor PbS nanocrystallites. Adv Mater 14(11):815–818. https://doi.org/10.1002/1521-4095(20020605)14:11<815::Aid-adma815>3.0.Co;2-k
Kundu D, Mondal S, Dutta D, Haque S, Ghosh AR (2016) Accumulation and contamination of lead in different trophic levels of food chain in sewage-fed East KolkataWetland,West Bengal, India. Int J Env Tech Sci 2:61–68
Kushwaha A, Hans N, Kumar S, Rani R (2018) A critical review on speciation, mobilization and toxicity of lead in soil microbe-plant system and bioremediation strategies. Ecotoxicol Environ Saf 147:1035–1045. https://doi.org/10.1016/j.ecoenv.2017.09.049
Li M, Cheng XH, Guo HX (2013) Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int Biodeterior Biodegrad 76:81–85. https://doi.org/10.1016/j.ibiod.2012.06.016
Li Z, Wang F, Bai T, Tao J, Guo J, Yang M, Wang S, Hu S (2016) Lead immobilization by geological fluorapatite and fungus Aspergillus niger. J Hazard Mater 320:386–392. https://doi.org/10.1016/j.jhazmat.2016.08.051
Liang J, Mao JS (2015) Source analysis of global anthropogenic lead emissions: their quantities and species. Environ Sci Pollut Res 22:7129–7138. https://doi.org/10.1007/s11356-014-3878-4
Liang X, Csetenyi L, Gadd GM (2016) Lead bioprecipitation by yeasts utilizing organic phosphorus substrates. Geomicrobiol J 33:294–307. https://doi.org/10.1080/01490451.2015.1051639
Lin WT, Huang Z, Li XZ, Liu MH, Cheng YJ (2016) Bio–remediation of acephate–Pb(II) compound contaminants by Bacillus subtilis FZUL-33. J Environ Sci 45:94–99. https://doi.org/10.1016/j.jes.2015.12.010
Lombardi PE, Peri SI, Verrengia NR (2010) ALA-D and ALA-D reactivated as biomarkers of lead contamination in the fish Prochilodus lineatus. Ecotoxicol Environ Saf 73:1704–1711
Lu J, Jiang YJ, Yu QS, Zou JW (2011) Molecular docking and molecular dynamics simulations of inhibitors binding to jack bean urease. Acta Chim Sin 69(20):2427–2433
Lyu JJ, Qin W, Zhang CH, Li FC (2020) Nanoparticle accumulation in microbial induced carbonate precipitation: the crucial role of extracellular polymeric substance. Geomicrobiol J 37:837–847. https://doi.org/10.1080/01490451.2020.1786866
Macaskie LE, Bonthrone KM, Yong P, Goddard DT (2000) Enzymically mediated bioprecipitation of uranium by a Citrobacter sp.: a concerted role for exocellular lipopolysaccharide and associated phosphatase in biomineral formation. Microbiology 146:1855–1867. https://doi.org/10.1099/00221287-146-8-1855
Mire CE, Tourjee JA, O’Brien WF et al (2004) Lead precipitation by Vibrio harveyi: evidence for novel quorum-sensing interactions. Appl Environ Microbiol 70(2):855–864. https://doi.org/10.1128/AEM.70.2.855-864.2004
Mitchell AC, Ferris FG (2005) The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: temperature and kinetic dependence. Geochim Cosmochim Acta 69:4199–4210. https://doi.org/10.1016/j.gca.2005.03.014
Mitra S, Pramanik K, Sarkar A, Ghosh PK, Soren T, Maiti TK (2018) Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol Environ Saf 156:183–196. https://doi.org/10.1016/j.ecoenv.2018.03.001
Morillo Perez JA, Garcia-Ribera R, Quesada T, Aguilera M, Ramos-Cormenzana A, Monteoliva-Sanchez M (2008) Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J Microbiol Biotechnol 24:2699–2704. https://doi.org/10.1007/s11274-008-9800-9
Mota R, Rossi F, Andrenelli L, Pereira SB, De Philippis R, Tamagnini P (2016) Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metalsbioremediation: interactions between metals and RPS binding sites. Appl Microbiol Biotechnol 100(17):7765–7775. https://doi.org/10.1007/s00253-016-7602-9
Mwandira W, Nakashima K, Kawasaki S, Ito M, Sato T, Igarashi T, Chirwa M, Banda K, Nyambe I, Nakayama S, Nakata H, Ishizuka M (2019) Solidification of sand by Pb(II)-tolerant bacteria for capping mine waste to control metallic dust: case of the abandoned Kabwe Mine, Zambia. Chemosphere 228:17–25. https://doi.org/10.1016/j.chemosphere.2019.04.107
Nriagu JO (1973) Lead orthophosphates—II. Stability of cholopyromophite at 25 °C. Geochim Cosmochim Acta 37:367–377. https://doi.org/10.1016/0016-7037(73)90206-8
Ohnuki T, Ozaki T, Yoshida T, Sakamoto F, Kozai N, Wakai E, Francis AJ, Iefuji H (2005) Mechanisms of uranium mineralization by the yeast Saccharomyces cerevisiae. Geochim Cosmochim Acta 69:5307–5316. https://doi.org/10.1016/j.gca.2005.06.023
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14:26. https://doi.org/10.3390/ijerph14121504
Park JH, Bolan N, Megharaj M, Naidu R (2011a) Comparative value of phosphate sources on the immobilization of lead, and leaching of lead and phosphorus in lead contaminated soils. Sci Total Environ 409:853–860. https://doi.org/10.1016/j.scitotenv.2010.11.003
Park JH, Bolan N, Megharaj M, Naidu R (2011b) Concomitant rock phosphate dissolution and lead immobilization by phosphate solubilizing bacteria (Enterobacter sp.). J Environ Manag 92:1115–1120. https://doi.org/10.1016/j.jenvman.2010.11.031
Pirbadian S, Barchinger SE, Leung KM, Byun HS, Jangir Y, Bouhenni RA, Reed SB, Romine MF, Saffarini DA, Shi L, Gorby YA, Golbeck JH, el-Naggar MY (2014) Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc Natl Acad Sci U S A 111:12883–12888. https://doi.org/10.1073/pnas.1410551111
Priyanka U, Gowda AKM, Elisha MG, Teja SB, Nitish N, Mohan RB (2017) Biologically synthesized PbS nanoparticles for the detection of arsenic in water. Int Biodeterior Biodegrad 119:78–86. https://doi.org/10.1016/j.ibiod.2016.10.009
Qian CX, Zhan QW (2016) Bioremediation of heavy metal ions by phosphate-mineralization bacteria and its mechanism. J Chin Chem Soc 63:635–639. https://doi.org/10.1002/jccs.201600002
Qian XY, Fang CL, Huang MS, Achal V (2017) Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. J Clean Prod 164:198–208. https://doi.org/10.1016/j.jclepro.2017.06.195
Rahman Z, Singh VP (2020) Bioremediation of toxic heavy metals (THMs) contaminated sites: concepts, applications and challenges. Environ Sci Pollut Res 27:27563–27581. https://doi.org/10.1007/s11356-020-08903-0
Rasulov BA, Yili A, Aisa HA (2013) Biosorption of metal ions by exopolysaccharide produced by Azotobacter chroococcum XU1. J Environ Prot 4:989–993
Ren GP, Sun Y, Ding Y, Lu AH, Li Y, Wang CQ, Ding HR (2018) Enhancing extracellular electron transfer between Pseudomonas aeruginosa PAO1 and light driven semiconducting birnessite. Bioelectrochemistry 123:233–240. https://doi.org/10.1016/j.bioelechem.2018.06.003
Rhee YJ, Hillier S, Pendlowski H, Gadd GM (2014) Pyromorphite formation in a fungal biofilm community growing on lead metal. Environ Microbiol 16:1441–1451. https://doi.org/10.1111/1462-2920.12416
Roeselers G, Van Loosdrecht M (2010) Microbial phytase-induced calcium-phosphate precipitation-a potential soil stabilization method. Folia Microbiol 55:621–624
Ruby MV, Davis A, Nicholson A (1994) In situ formation of lead phosphates in soils as a method to immobilize lead. Environ Sci Technol 28:646–654. https://doi.org/10.1021/es00053a018
Sakata K, Sakaguchi A, Tanimizu M, Takaku Y, Yokoyama Y, Takahashi Y (2014) Identification of sources of lead in the atmosphere by chemical speciation using X-ray absorption near-edge structure (XANES) spectroscopy. J Environ Sci 26:343–352. https://doi.org/10.1016/s1001-0742(13)60430-1
Sammut ML, Noack Y, Rose J, Hazemann JL, Proux O, Depoux M, Ziebel A, Fiani E (2010) Speciation of Cd and Pb in dust emitted from sinter plant. Chemosphere 78:445–450. https://doi.org/10.1016/j.chemosphere.2009.10.039
Sari A, Tuze M, Uluozlu OD, Soylak M (2007) Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem Eng J 37:151–158. https://doi.org/10.1016/j.bej.2007.04.007
Sayer JA, Cotter-Howells JD, Watson C, Hillier S, Gadd GM (1999) Lead mineral transformation by fungi. Curr Biol 9:691–694. https://doi.org/10.1016/s0960-9822(99)80309-1
Schwab AP, He YH, Banks MK (2005) The influence of organic ligands on the retention of lead in soil. Chemosphere 61:856–866
Seshadri S, Saranya K, Kowshik M (2011) Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast, Rhodosporidium diobovatum. Biotechnol Prog 27:1464–1469. https://doi.org/10.1002/btpr.651
Shahid M, Dumat C, Aslam M, Pinelli E (2012) Assessment of lead speciation by organic ligands using speciation models. Chem Speciat Bioavailab 24:248–252. https://doi.org/10.3184/095422912x13495331697627
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141. https://doi.org/10.1016/j.jcis.2004.01.036
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of CaCO3. Soil Biol Biochem 31:1563–1571. https://doi.org/10.1016/s0038-0717(99)00082-6
Su YQ, Zhao YJ, Zhang WJ, Chen GC, Qin H, Qiao DR, Chen YE, Cao Y (2020) Removal of mercury(II), lead(II) and cadmium(II) from aqueous solutions using Rhodobacter sphaeroides SC01. Chemosphere 243(7):125166. https://doi.org/10.1016/j.chemosphere.2019.125166
Subbaiah MV, Yuvaraja G, Vijaya Y, Krishnaiah A (2011) Equilibrium, kinetic and thermodynamic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by fungus (Trametes versicolor) biomass. J Taiwan Inst Chem Eng 42:965–971. https://doi.org/10.1016/j.jtice.2011.04.007
Sun YB, Li Y, Xu YM, Liang XF, Wang L (2015) In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl Clay Sci 105:200–206. https://doi.org/10.1016/j.clay.2014.12.031
Sun X, Han F, Wang H, Song FP, Cui XM, Lou YH, Zhuge YP (2018) Characterization of three Pb-resistant fungi and their potential Pb2+ ions adsorption capacities. Water Sci Technol 78:2616–2625. https://doi.org/10.2166/wst.2019.019
Tahir M, Khalid U, Ijaz M, Shah GM, Naeem MA, Shahid M, Mahmood K, Ahmad N, Kareem F (2018) Combined application of bio-organic phosphate and phosphorus solubilizing bacteria (Bacillus strain MWT 14) improve the performance of bread wheat with low fertilizer input under an arid climate. Braz J Microbiol 49:15–24. https://doi.org/10.1016/j.bjm.2017.11.005
Tang JY, Zhang J, Ren L, Zhou Y, Gao J, Luo L, Yang Y, Peng Q, Huang H, Chen A (2019) Diagnosis of soil contamination using microbiological indices: a review on heavy metal pollution. J Environ Manag 242:121–130. https://doi.org/10.1016/j.jenvman.2019.04.061
Teng ZD, Shao W, Zhang KY, Huo YQ, Zhu J, Li M (2019) Pb biosorption by Leclercia adecarboxylata: protective and immobilized mechanisms of extracellular polymeric substances. Chem Eng J 375:10. https://doi.org/10.1016/j.cej.2019.122113
Tian D, Jiang Z, Jiang L, Su M, Feng Z, Zhang L, Wang S, Li Z, Hu S (2019) A new insight into lead (II) tolerance of environmental fungi based on a study of Aspergillus niger and Penicillium oxalicum. Environ Microbiol 21:471–479. https://doi.org/10.1111/1462-2920.14478
Turner A, Lewis M (2018) Lead and other heavy metals in soils impacted by exterior legacy paint in residential areas of south west England. Sci Total Environ 619:1206–1213. https://doi.org/10.1016/j.scitotenv.2017.11.041
Van Wyk CS (2011) Removal of heavy metals from metal-containing effluent by yeast biomass. Afr J Biotechnol 10(55):11557–11561
Wang JL, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451. https://doi.org/10.1016/j.biotechadv.2006.03.001
Wang JL, Chen C (2010) Research advances in heavy metal removal by biosorption. Acta Sci Circumst 30(4):673–701
Wang JL, Zhan XM, Ding DC, Zhou D (2001) Bioadsorption of lead(II) from aqueous solution by fungal biomass of Aspergillus niger. J Biotechnol 87:273–277. https://doi.org/10.1016/s0168-1656(00)00379-5
Wang XH, Zhao CX, Pan XL (2015) Bioremediation of Pb-pollution based on microbially induced calcite precipitation. Earth and Environment 43:80–85. https://doi.org/10.14050/j.cnki.1672-9250.2015.01.011
Wang MC, Hao RX, Ding Y (2017) Response mechanism of electric current on adsorption and immobilization of lead by Pencillium polonicum. Acta Petrol Mineral 36:858–864
Wei YQ, Zhao Y, Shi M, Cao Z, Lu Q, Yang T, Fan Y, Wei Z (2018) Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour Technol 247:190–199. https://doi.org/10.1016/j.biortech.2017.09.092
Wheaton G, Counts J, Mukherjee A, Kruh J, Kelly R (2015) The confluence of heavy metal biooxidation and heavy metal resistance: implications for bioleaching by extreme thermoacidophiles. Minerals 5:397–451. https://doi.org/10.3390/min5030397
Wu F, Hao RX, Lu AH, Jiang Y, Yang S (2015) Biological characteristics of Aspergillus tubingensis and its fixation to Pb2+. Acta Sci Circumst 35:144–151
Xu Y, Qian C, Lu Z (2013) Remediation of heavy metal contaminated soils by bacteria biomineralization. J Environ Eng 7:2763–2768
Xu XY, Hao RX, Xu H, Lu AH (2020) Removal mechanism of Pb(II) by Penicillium polonicum: immobilization, adsorption, and bioaccumulation. Sci Rep 10(1):9079. https://doi.org/10.1038/s41598-020-66025-6
Yang XF, Shen ZG, Zhang B, Yang JP, Hong WX, Zhuang ZX, Liu JJ (2013) Silica nanoparticles capture atmospheric lead: implications in the treatment of environmental heavy metal pollution. Chemosphere 90:653–656. https://doi.org/10.1016/j.chemosphere.2012.09.033
Yari M, Rajabi M, Moradi O, Yari A, Asif M, Agarwal S, Gupta VK (2015) Kinetics of the adsorption of Pb(II) ions from aqueous solutions by graphene oxide and thiol functionalized graphene oxide. J Mol Liq 209:50–57. https://doi.org/10.1016/j.molliq.2015.05.022
Yousaf B, Liu GJ, Abbas Q, Ullah H, Wang RW, Zia-ur-Rehman M, Amina NZY (2018) Comparative effects of biochar-nanosheets and conventional organic-amendments on health risks abatement of potentially toxic elements via consumption of wheat grown on industrially contaminated-soil. Chemosphere 192:161–170. https://doi.org/10.1016/j.chemosphere.2017.10.137
Zeng GM, Li NJ, Huang DL, Lai C, Zhao MH, Huang C, Wei Z, Xu P, Zhang C, Cheng M (2015) The stability of Pb species during the Pb removal process by growing cells of Phanerochaete chrysosporium. Appl Microbiol Biotechnol 99:3685–3693. https://doi.org/10.1007/s00253-014-6275-5
Zhang W, Huang Y (2020) The synthesis of PbS NPs and biosorption of Pb(II) by Shinella zoogloeoides PQ7 in aqueous conditions. Water 12(7):2065. https://doi.org/10.3390/w12072065
Zhang XW, Yang LS, Li YH, Li HR, Wang WY, Ge QS (2011) Estimation of lead and zinc emissions from mineral exploitation based on characteristics of lead/zinc deposits in China. Trans Nonferrous Metals Soc China 21:2513–2519. https://doi.org/10.1016/s1003-6326(11)61044-3
Zhang ML, Wang HX, Han XM (2016) Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment. Chemosphere 154:215–223. https://doi.org/10.1016/j.chemosphere.2016.03.103
Zhang KJ, Xue YW, Xu HH, Yao YN (2019) Lead removal by phosphate solubilizing bacteria isolated from soil through biomineralization. Chemosphere 224:272–279
Zhang C, Li X, Lyu J, Li F (2020) Comparison of carbonate precipitation induced by Curvibacter sp. HJ-1 and Arthrobacter sp. MF-2: further insight into the biomineralization process. J Struct Biol:107609. https://doi.org/10.1016/j.jsb.2020.107609
Zhao XM, Do H, Zhou Y, Li Z, Zhang XF, Zhao SJ, Li MT, Wu D (2019) Rahnella sp. LRP3 induces phosphate precipitation of Cu (II) and its role in copper-contaminated soil remediation. J Hazard Mater 368:133–140. https://doi.org/10.1016/j.jhazmat.2019.01.029
Zhou GT, Xia X, Wang H, Li LQ, Wang GJ, Zheng SX, Liao SJ (2016) Immobilization of lead by Alishewanella sp WH16-1 in pot experiments of Pb-contaminated paddy soil. Water Air Soil Pollut 227:11. https://doi.org/10.1007/s11270-016-3040-7
Zhu XL, Lv BX, Shang XQ, Wang JQ, Li M, Yu XY (2019) The immobilization effects on Pb, Cd and Cu by the inoculation of organic phosphorus-degrading bacteria (OPDB) with rapeseed dregs in acidic soil. Geoderma 350:1–10. https://doi.org/10.1016/j.geoderma.2019.04.015
Acknowledgments
We are grateful for grants from the National Natural Science Foundation of China (41672332), National Key Research and Development Project of China (2019YFC1805901), and National Basic Research Program of China (2014CB846003). We thank all our colleagues and students who were involved in this work for their unremitting efforts. We also thank all the editors and anonymous reviewers for helpful comments on this manuscript.
Funding
This study was part-funded by the National Natural Science Foundation of China (41672332), National Key Research and Development Project of China (2019YFC1805901), and National Basic Research Program of China (2014CB846003).
Author information
Authors and Affiliations
Contributions
Bing Shan: Investigation; writing-original draft; writing-review and editing
Ruixia Hao: Writing-review and editing; paper administration.
Hui Xu, Jiani Li, Yinhuang Li, Xiyang Xu, and Junman Zhang: Investigation and data collection
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shan, B., Hao, R., Xu, H. et al. A review on mechanism of biomineralization using microbial-induced precipitation for immobilizing lead ions. Environ Sci Pollut Res 28, 30486–30498 (2021). https://doi.org/10.1007/s11356-021-14045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14045-8