Abstract
After the primary structure of P2X receptors had been identified, their function had to be characterized on the molecular level. Since these ligand-gated ion channels become activated very quickly after binding of ATP, methods with adequate time resolution have to be applied to investigate the early events induced by the agonist. Single-channel recordings were performed to describe conformational changes on P2X2, P2X4, and P2X7 receptors induced by ATP and also by allosteric receptor modifiers. The main results of these studies and the models of P2X receptor kinetics derived from these observations are reviewed here. The investigation of purinoceptors by means of the patch clamp technique following site-directed mutagenesis will probably reveal more details of P2X receptor function at the molecular level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Primarily, P2X receptors are ligand-gated ion channels. This ion channel family, also known as ion channel-coupled receptors, mediates the fastest known signal transduction from extracellular messengers to the intracellular environment. This signal transduction mechanism is so simple (i.e., includes few steps) that it becomes fully activated in the low millisecond range. The best known biological structures using this approach are neuronal synapses in which receptors for the classic transmitters acetylcholine, glutamate, gamma-aminobutyric acid (GABA), glycine, or serotonin transmit excitation from one neuron to another in less than 1 ms [1]. This rapid activation kinetics of the ligand-gated ion channels is too fast to become analyzed by whole-cell current recordings or even slower fluorescent dye-based measurements of changes of the intracellular ion composition. The main reason for the low speed of these recording techniques compared to the receptor kinetics is the rate-limiting solution exchange at the extracellular side of the cell membrane where the ligand binding site is located. These obstacles in the investigation of ligand-gated channels can be overcome by the single-channel patch clamp technique where tiny parts (patches) are excised out of the cell membrane containing at best only one receptor. In such preparations, solution exchanges can be achieved within less than 1 ms [2]. Apart from the high time resolution, this technique has a resolution of ionic currents in the pA (picoampere) range sufficient to observe the ionic flow through and the gating (opening and closing) of single ion channels. With this technique, ion channels can be characterized and identified according to their conductance, macroscopic kinetics (time course of activation, i.e., opening of channels after ATP application, and of deactivation, i.e., closing of channels after ATP withdrawal), and microscopic kinetics (i.e., the characteristic mean times channels spend in closed or open configurations). The ultimate goal of the kinetic analysis is the development of a kinetic (normally Markovian) model of closed and open states which describes the agonist concentration dependency of the macroscopic and microscopic kinetics. Additionally, single-channel analysis may be able to discriminate whether various effects of agonists and antagonists are caused by changing agonist binding or efficacy or by altering the ion channel permeation behavior [3].
Additionally, patch clamping allows the separation of currents flowing through the ligand-gated channels from contaminating currents mediated by conductances activated downstream by secondary signals. In the case of P2X receptors, such signals may be changes in the intracellular concentration of Ca2+, Na+, K+, or H+ ions which can permeate the channel pore. The next steps in signal cascades may be changes of the cell volume with effects on the cytoskeleton, oxidative stress, and furthermore activation of kinases, phosphatases, or transcription. Therefore, investigating the time course of opening and closing of P2X receptors may help to understand signaling cascades they activate. It may also detect functional changes induced by receptor mutations more precisely than whole-cell current recordings or measurements of P2X receptor-induced changes of the intracellular Ca2+ concentration or even later effects.
Soon after the introduction of the patch clamp technique, ATP-activated single channel events were discovered and analyzed. They were first described in rat sensory neurons [4] and cultured chicken myoblasts [5]. Later, different ATP-activated single ion channel currents were reported in smooth and cardiac muscle, glands, and other neurons where single-channel conductances between <1 and 60 pS were measured [6].
More detailed information about P2X receptor kinetics was obtained from measurements of the rise time of ATP-induced whole-cell currents which, as typical for ligand-gated receptors, decreased with increasing agonist concentration in the range of 5–25 ms [7, 8]. A more sophisticated kinetic analysis of P2X receptor function was performed in bullfrog dorsal root ganglion cells. The concentration dependence of the activation and deactivation time course as well as of the steady-state current during prolonged ATP application could well be described by a linear Markov model where the independent binding of three ATP molecules to equal binding sites leads to the opening of the channel which in the triliganded state is open 25% of the time:

Here, the rate constants are given in s−1 and β/α = 1/4 [9]. Remarkably, the trimeric structure of cloned P2X receptor channels described much later [10–12] implies the necessity of binding of three ATP molecules and therefore coincides well with this model.
Investigations of the microscopic kinetics of native P2X receptors were used to characterize aspects of the gating behavior of the channels in distinct preparations but were not developed into kinetic models [5, 13–18].
After cloning the P2X receptor subtypes, the kinetic analysis of heterologously expressed distinct P2X receptors became principally possible. However, the collection of enough single-channel data for establishing a kinetic model turned out to be too laborious or even seemed to be impossible on receptors which desensitize quickly (within milliseconds) and recover slowly (within minutes) [19, 20]. Therefore, models for P2X1 [21] and P2X3 [22] receptors have been developed which, like the model developed by Bean [9], are based on whole-cell recording and include additional desensitized receptor states.
For the slowly desensitizing P2X4 receptor, the effect of external Mg2+ ions was investigated on the single-channel level. The blocking effect of Mg2+ could be partially ascribed to the reduction of the mean open time [23]. A more detailed analysis of the single-channel kinetics of P2X4 receptors revealed three different mean open times and five mean closed times following activation of the receptor by 0.3 μM ATP, where desensitization is slow. Hence, it was concluded that a kinetic model should have at least three open and five closed states [24].
A more comprehensive investigation of the single-channel kinetics under steady-state conditions (i.e., during prolonged application of constant extracellular ATP concentrations where the distribution of conformational states of receptors is not changing with time) was performed on the very slowly desensitizing P2X2 receptor [25]. It should, however, be mentioned that P2X2 receptors may exhibit substantial fast desensitization in excised patches [26]. The following model was developed:

In contrast to Bean’s model [9] the three ATP binding sites are not equal in this case. Specifically, the second binding step possesses a higher association rate constant and a much higher dissociation rate constant compared to the first. Both together result in a decreased affinity. This could be interpreted in such a way that the binding of ATP at the first site induces a conformation change that leads to an easier accessibility of a second binding to ATP. But this second binding is weaker, possibly due to the repulsion of both anionic ATP4− molecules. Negative cooperativity due to ATP repulsion was also suggested for the human P2X7 receptor [27]. The addition of an open (O6) and a closed state (C7) to a simple C–C–C–C–O model was necessary to describe openings of the P2X2 receptor which occurred as single openings or as bursts. This model was however unable to explain P2X4 receptor kinetics of multichannel patches, and therefore a positive cooperativity toward opening of P2X4 receptor channels was assumed [28].
The P2X7 is another non-desensitizing P2X receptor which proved to be suitable for kinetic analysis [29]. A relatively simple model was used to describe both the ATP dependence of microscopic (open and shut times, open probability of single channels) and macroscopic kinetics (activation and deactivation time course) of the main gating mode measured by a combination of single-channel recording and an ultrafast solution exchange system:

The model could be simplified compared to that describing the kinetics of the P2X2 receptor, since the open time distribution could be described in most cases by one exponential component and the channel did not display a bursting behavior. The much lower association rate constants compared to the P2X2 model are owed to the low ATP affinity of the P2X7 receptor. The kinetics of a second gating mode of single P2X7 receptors were about four times slower but could principally also be described by this model.
The assumption of only two ATP binding sites was sufficient for the approximation of the measured P2X7 receptor kinetics. This is a further deviation from the models mentioned above. The finding that P2X7 receptors are putative trimers like other P2X receptors and therefore contain probably three ATP binding sites seems to be contradictory to the P2X7 receptor kinetics model. As yet, a comprehensive explanation cannot be given but there are indications for a third binding site that induces very small single ion channel currents (Riedel et al., unpublished).
Mean currents calculated by this model displayed an approximately monoexponential activation and deactivation time course with time constants in the range of 10–25 ms. It was much simpler than the kinetics of several measured P2X7 receptor-dependent whole-cell currents [11]. This indicates that under whole-cell conditions either other ion currents may become secondarily activated or the biophysical properties of single P2X7 receptors themselves become less complex due to washout of an essential receptor component. However, even in cell-attached recordings, in which the intracellular environment is preserved, the single-channel current measurements at P2X7 receptors gave indication neither of more complex activation kinetics nor of a dilatation of the channel pore which is assumed to occur during prolonged ATP application [11]. Moreover, these single-channel current characteristics are indistinguishable from those measured in human lymphocytes [16], corroborating the view that the native human P2Z receptor is a genuine P2X7 receptor. Detailed analysis of the permeation characteristics [30] revealed a stable single-channel conductance even in external Na+-free and low Ca2+ solutions during long-lasting application of large ATP concentrations where a permeability of the cell membrane to large organic cations was induced in whole-cell preparations [11, 31, 32]. Instead, the permeation behavior and the calculated pore size of about 8.5 Å was similar to P2X1 and P2X2 receptors [33, 34]. Accordingly, it can be concluded that the apparent pore dilatation of P2X7 receptors observed in macroscopic current recordings [32] has no equivalent at the single-channel level, suggesting that the P2X7 receptor-induced permeability increase is not due to pore dilatation but reflects the induction of additional conductances mediated by other proteins secondary to P2X7 receptor activation. As recently reported, such additional conductance may be established by pannexin [35, 36], which can be activated by intracellular Ca2+ ions [37] and membrane stretch [38]. Similarly, single-channel recordings from P2X2 [33] and P2X4 receptors [24] do not indicate any pore dilatation which is assumed to occur in whole-cell preparation [39–41].
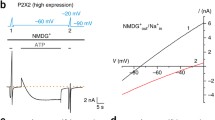
Instead of changing the permeation behavior, extracellular Na+ removal was found to drastically change the P2X7 ion channel kinetics [30]. The open probability, the mean open time as well as the activation and deactivation time constants considerably increased when Na+ was replaced by other monovalent cations. This effect was highly specific to Na+, suggesting that Na+ is not only a permeating ion but also a modulator of P2X7 receptor gating. The fact that the P2X7 receptor-induced increase of the cell membrane permeability to organic cations is promoted in Na+-free media [42, 43] can therefore be explained by an enhanced P2X7 receptor activation. This results in an increased Na+ and Ca2+ influx that may activate further permeation pathways.
It turned out that the kinetic model for P2X7 receptors [29] could easily explain the potentiating effect of Na+ removal, assuming that binding of Na+ to an extracellular site reduces the rate constant from O4 to C3 from 200 s−1 to 8 s−1. Although this is an allosteric effect not interfering with ATP binding, the concentration-response curve is shifted leftwards by Na+ removal, giving rise to a reduced apparent ATP binding constant. Such an apparent increase of a binding site affinity due to an increased efficacy has already been described by Colquhoun [44]. In this respect, single-channel recording has again proven to be the (possibly only) method to quantitatively measure and distinguish effects on single proteins of drug binding and the following conformational changes considered as efficacy. The model explains on the molecular level the known stimulating effect of Na+ substitution on effects mediated by P2X7 or P2X7-like native receptors [11].
Under conditions of hypoxia or tissue injury, ATP and K+ are secreted into the extracellular space [11]. Therefore, in metabolically compromised tissues, a replacement of extracellular Na+ by K+ may enhance the efficacy and potency of ATP on P2X7 receptors. Furthermore, ATP-mediated cell depolarization increases the driving force for K+ efflux and lowers the EC50 value of Na+ since the site seems to be located in the electrical field of the membrane [30]. In this way, K+ efflux may reinforce the ATP effect on P2X7 receptor-expressing cells, and even low concentrations of ATP may activate the P2X7 receptor to a substantial extent. However, whether such a mechanism is of relevance in vivo remains to be established.
In the future, the combination of single-channel recordings and molecular engineering methods will reveal more details concerning the function of P2X receptor-dependent ion channels. To this end, it may help to dissect and understand early and late steps of the signaling cascades which are started with the binding of ATP and are followed by biological processes known to be influenced by P2X receptors like contraction, secretion, perception, proliferation, and apoptosis [45]. Furthermore, patch clamp recording will continue to describe the effects of P2X receptor modulators on the single-channel level (for review of the investigations already performed, see North [11]).
References
Hille B (2001) Ion channels of excitable membranes. Sinauer, Sunderland, MA
Sakmann B, Neher E (1995) Single channel recording. Plenum, New York
Colquhoun D (2006) Agonist-activated ion channels. Br J Pharmacol 147:S17–S26
Krishtal OA, Marchenko SM, Pidoplichko VI (1983) Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett 35:41–45
Kolb HA, Wakelam MJO (1983) Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature 303:621–623
Bean BP (1992) Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci 13:87–90
Bean BP, Friel D (1990) ATP-activated channels in excitable cells. In: Narahashi T (ed) Ion channels, 2nd edn. Plenum, New York, pp 169–203
Surprenant A (1996) Functional properties of native and cloned P2X receptors. In: Chadwick DJ, Goode JA (eds) P2 purinoceptors: localization, function and transduction mechanisms. Ciba Foundation Symposium 198. Wiley, Chichester, pp 208–222
Bean BP (1990) ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci 10:1–10
Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17:3016–3028
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM (2005) Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem 280:10759–10765
Krishtal OA, Marchenko SM, Obukhov AG (1988) Cationic channels activated by extracellular ATP in rat sensory neurons. Neurosci Lett 27:995–1000
Cloues R (1995) Properties of ATP-gated channels recorded from rat sympathetic neurons: voltage dependence and regulation by Zn2+ ions. J Neurophysiol 73:312–319
Wright JM, Li CY (1995) Zn2+ potentiates steady-state ATP activated currents in rat nodose ganglion neurons by increasing the burst duration of a 35 pS channel. Neurosci Lett 193:177–180
Markwardt F, Löhn M, Böhm T, Klapperstück M (1997) Purinoceptor-operated cationic channels in human B lymphocytes. J Physiol (Lond) 498:143–151
Ugur M, Drummond RM, Zou H, Sheng PH, Singer JJ, Walsh JV (1997) An ATP-gated cation channel with some P2Z-like characteristics in gastric smooth muscle cells of toad. J Physiol (Lond) 498:427–442
Wong AYC, Burnstock G, Gibb AJ (2000) Single channel properties of P2X ATP receptors in outside-out patches from rat hippocampal granule cells. J Physiol (Lond) 527:529–547
Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G (1994) A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature 371:516–519
Evans RJ (1996) Single channel properties of ATP-gated cation channels (P2X receptors) heterologously expressed in Chinese hamster ovary cells. Neurosci Lett 212:212–214
Rettinger J, Schmalzing G (2003) Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol 121:451–461
Sokolova E, Skorinkin A, Moiseev I, Agrachev A, Nistri A, Giniatullin R (2006) Experimental and modeling studies of desensitization of P2X3 receptors. Mol Pharmacol 70:373–382
Negulyaev YA, Markwardt F (2000) Block by extracellular Mg2+ of single human purinergic P2X4 receptor channels expressed in human embryonic kidney cells. Neurosci Lett 279:165–168
Priel A, Silberberg SD (2004) Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol 123:281–293
Ding SH, Sachs F (1999) Single channel properties of P2X2 purinoceptors. J Gen Physiol 113:695–719
Ding SH, Sachs F (2000) Inactivation of P2X2 purinoceptors by divalent cations. J Physiol (Lond) 522:199–214
Klapperstück M, Büttner C, Schmalzing G, Markwardt F (2001) Functional evidence of distinct ATP activation sites at the human P2X7 receptor. J Physiol (Lond) 534:25–35
Ding SH, Sachs F (2002) Evidence for non-independent gating of P2X2 receptors expressed in Xenopus oocytes. BMC Neurosci 3:17
Riedel T, Lozinsky I, Schmalzing G, Markwardt F (2007) Kinetics of P2X7 receptor-operated single channels currents. Biophys J 92:2377–2391
Riedel T, Schmalzing G, Markwardt F (2007) Influence of extracellular monovalent cations on pore and gating properties of P2X7 receptor-operated single channels currents. Biophys J 93:846-858. DOI 10.1529/biophysj.106.103614
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738
Virginio C, MacKenzie A, North RA, Surprenant A (1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol (Lond) 519:335–346
Ding S, Sachs F (1999) Ion permeation and block of P2X2 purinoceptors: single channel recordings. J Membr Biol 172:215–223
Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA (1996) Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol (Lond) 497:413–422
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1 release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082
Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett 581:483–488
Locovei S, Wang JJ, Dahl G (2006) Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580:239–244
Bao L, Locovei S, Dahl G (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572:65–68
Egan TM, Samways DSK, Li ZY (2006) Biophysics of P2X receptors. Pflugers Arch 452:501–512
Khakh BS, Bao XR, Labarca C, Lester HA (1999) Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci 2:322–350
Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A (1999) Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2:315–321
Jiang LH, Rassendren F, MacKenzie A, Zhang YH, Surprenant A, North RA (2005) N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X7 receptors. Am J Physiol 289:C1295–C1302
Fernando KC, Gargett CE, Wiley JS (1999) Activation of the P2Z/P2X7 receptor in human lymphocytes produces a delayed permeability lesion: involvement of phospholipase D1. Arch Biochem Biophys 362:197–202
Colquhoun D (1998) Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol 125:924–947
Khakh BS, North RA (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527–532
Acknowledgement
This work was supported by grants of the Deutsche Forschungsgemeinschaft (Ma1581/12-1) and the Roux-programme of the Medical Faculty of the Martin Luther University Halle (Roux 5/09, 10/01 and 13/07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Markwardt, F. Activation kinetics of single P2X receptors. Purinergic Signalling 3, 249–253 (2007). https://doi.org/10.1007/s11302-007-9070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-007-9070-2