Abstract
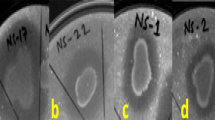
A total of 72 isolates of root-associated/endophytic (RAE) bacteria were isolated from peanut plants grown in the main producing areas of 6 provinces in China. The 16S rRNA gene sequences of these isolates were determined and phylogenetic analyses revealed that 72 isolates belonged to the classes Bacilli (49 isolates) and Gammaproteobacteria (23 isolates). The majority of RAE bacteria in Bacilli belonged to 2 genera, Bacillus and Lysinibacillus (48 and 1) while those in Gammaproteobacteria belonged to the genera Enterobacter, Serratia, Stenotrophomonas, and Pseudomonas (7, 11, 3 and 2 isolates, respectively). This is the first report of Lysinibacillus xylanilyticus isolate as biocontrol agent against AFs. All of the selected RAE bacteria showed inhibitory activities against Aspergillus parasiticus (A. parasiticus) growth and/or aflatoxins (AFs) production by visual agar plate assay and tip culture method. Most of the RAE bacteria strains (96 % strains) were determined to have decreased mycelia growth or AFs production levels by >50 % (p < 0.05). Bacterial isolates were further characterized for chitinolytic activity and 22 strains (30 % strains) of identified RAE bacteria degraded colloidal chitin on the chitin medium plate. Ten selected chitinolytic RAE bacteria were tested for antifungal activity on peanuts and most of them significantly decreased mycelial growth and AFs production levels by >90 %. These results showed a wide distribution of biological control bacteria against AFs in Chinese peanut main producing areas and the selected RAE bacteria could potentially be utilized for the biocontrol of toxicogenic fungi.





Similar content being viewed by others
References
Ahmed I, Yokota A, Yamazoe A, Fujiwara T (2007) Proposal of Lysinibacillus boronitolerans gen. nov., sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int J Syst Evol Microbiol 57:1117–1125
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Batista LR, Chalfoun SM, Prado G, Schwan RF, Wheals AE (2003) Toxigenic fungi associated with processed (green) coffee beans (Coffea arabica L.). Int J Food Microbiol 85:293–300
Bayman P, Baker JL, Mahoney NE (2002) Aspergillus on tree nuts: incidence and associations. Mycopathologia 155:161–169
Brown RL, Cotty PJ, Cleveland TE (1991) Reduction in aflatoxin content of maize by atoxigenic strains of Aspergillus flavus. J Food Prot 54:623–626
Burr TJ, Matteson MC, Smith CA, Corral-Garcia MR, Huang TC (1996) Effectiveness of bacteria and yeasts from apple orchards as biological control agents of apple scab. Biol Control 6:151–157
Cotty PJ (1994) Influence of field application of an atoxigenic strain of Aspergillus flavus on the population of A. flavus infecting cotton bolls and on the aflatoxin content of cottonseed. Phytopathology 84:1270–1277
Cotty PJ, Lee LS (1989) Aflatoxin contamination of cottonseed: comparison of pink bollworm damaged and undamaged bolls. Troph Sci 29:273–276
Diener UL, Cole RJ, Sanders TH, Payne GA, Lee LS, Klich MA (1987) Epidemiology of aflatoxin formation by Aspergillus flavus. Annu Rev Phytopathol 25:249–270
Dorner JW, Cole RJ, Blankenship PD (1992) Use of a biocompetitive agent to control preharvest aflatoxin in drought stressed peanuts. J Food Prot 55:888–892
Dorner JW, Cole RJ, Blankenship PD (1998) Effect of inoculum rate of biocontrol agents on preharvest aflatoxin contamination of peanuts. Biol Control 12:171–176
Dorner JW, Cole RJ, Wicklow DT (1999) Aflatoxin reduction in corn through field application of competitive fungi. Food Prot 62:650–656
Edwards U, Rogall T, Blocker H, Emde M, Bottger EC (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucl Acids Res 17(19):7843–7853
Egmond HP (1995) Mycotoxins: regulations, quality assurance and reference materials. Food Addit Contam 12:321–330
Horn BW, Dorner JW, Greene RL, Blankenship PD, Cole RJ (1994) Effect of Aspergillus parasiticus soil inoculum on invasion of peanut seeds. Mycopathologia 125:179–191
Hua ST, Grosjean OK, Baker JL (1999) Inhibition of aflatoxin biosynthesis by phenolic compounds. Lett Appl Microbiol 29:289–291
Huang CJ, Wang TK, Chung SC, Chen CY (2005) Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28–9. J Biochem Mol Biol 38:82–88
Jeounghyun R, Madhaiyan M, Oonguzhali S, Yim W, Indiragandhi P, Kim K, Anandham R, Yun J, Kim KH, Sa T (2006) Plant growth substances produced by methylobacterium spp. and their effect on tomato (Lycopersicon esculentum L.) and red pepper (Capsicum annuum L.) growth. J Microbiol Biotechnol 16(10):1622–1628
Kerry BR (2000) Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu Rev Phytopathol 38:423–441
Klich MA (2007) Aspergillus flavus: the major producer of aflatoxin. Mol Plant Pathol 8:713–722
Kondo T, Sakurada M, Okamoto S, Ono M, Tsukigi H, Suzuki A, Nagasawa H, Sakuda S (2001) Effects of aflastatin A, an inhibitor of aflatoxin production, on aflatoxin biosynthetic pathway and glucose metabolism in Aspergillus parasiticus. J Antibiot (Tokyo) 54:650–657
Kong Q, Shan S, Liu Q, Wang X, Yu F (2010) Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int J Food Microbiol 139:31–35
Lee CS, Jung YT, Park S, Oh TK, Yoon JH (2010) Lysinibacillus xylanilyticus sp. nov., a xylandegrading bacterium isolated from forest humus. Int J Syst Evol Microbiol 60:281–286
Miwa H, Ahmed I, Yokota A, Fujiwara T (2009) Lysinibacillus parviboronicapiens sp. nov., a low-boron-containing bacterium isolated from soil. Int J Syst Evol Microbiol 59:1427–1432
Monreal J, Reese ET (1969) The chitinase of Serratia marcestens. Can J Microbial 15(7):689
Moyne AL, Shelby R, Cleveland TE, Tuzun S (2001) Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J Appl Microbiol 90:622–629
Munimbazi C, Bullerman LB (1997) Inhibition of aflatoxin production of Aspergillus parasiticus NRRL 2999 by Bacillus pumilus. Mycopathologia 140:163–169
Nesci AV, Bluma RV, Etcheverry MG (2005) In vitro selection of maize rhizobacteria to study potential biological control of Aspergillus section Flavi and aflatoxin production. Eur J Plant Pathol 113(2):159–171
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572
Nielsen PV, Rios R (2000) Inhibition of fungal growth on bread by volatile components from spices and herbs, and possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol 60:219–229
Park DL (1993) Controlling aflatoxin in food and feed. Food Technol 47:92–96
Rao GP, Sigh HN (1992) Fungitoxic evaluation of essential oils extracted from higher plants against some sugarcane pathogens in vitro. Trop Sci 32:377–382
Roberts WK, Selitrennikoff CP (1988) Plant and bacterial chitinases differ in antifungal activity. Gen Microbiol 61:1720–1726
Someya N, Kataoka N, Komagata T, Hirayae K, Hibi T, Akutsu K (2000) Biological control of cyclamen soilborne diseases by Serratia marcescens strain B2. Plant Dis 84:334–340
Someya N, Nakajima M, Hirayae K, Hibi T, Akutsu K (2001) Synergistic antifungal activity of chitinolytic enzymes and prodigiosin produced by biocontrol bacterium, Serratia marcescens strain B2 against gray mold pathogen Botrytis cinerea. J Gen Plant Pathol 67:312–317
Someya N, Nakajima M, Watanabe K, Hibi T, Akutsu K (2005) Potential of Serratia marcescens strain B2 for biological control of rice sheath blight. Biocontrol Sci Technol 15:105–109
Someya N, Ikeda S, Morohoshi T, Tsujimoto MN, Yoshida T, Sawada H, Ikeda T, Tsuchiya K (2011) Diversity of culturable chitinolytic bacteria from rhizospheres of agronomic plants in Japan. Microbes Environ 26(1):7–14
Sweeney MJ, Dobson AD (1998) Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int J Food Microbiol 43:141–158
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA 4: molecularevolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Taylor WJ, Draughon FA (2001) Nannocystis exedens: a potential biocompetitive agent against Aspergillus flavus and Aspergillus parasiticus. J Food Prot 64:1030–1034
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wu F, Khlangwiset P (2010) Health economic impacts and cost-effectiveness of aflatoxin reduction strategies in Africa: case studies in biocontrol and postharvest interventions. Food Addit Contam 27:496–509
Yan PS, Song Y, Sakuno E, Nakajima H, Nakagawa H, Yabe K (2004) Cyclo(L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl Environ Microbiol 70:7466–7473
Yan-ni YIN, Lei-yan YAN, Jin-hua JIANG, Zhong-hua MA (2008) Biological control of aflatoxin contamination of crops. J Zhejiang Univ Sci B 9(10):787–792
Zhang Z, Yuen GY, Sarath G, Penheiter AR (2001) Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology 91:204–211
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 30571244 and No. 30870003) and Project(HIT-NSRIF200806)supported by Natural Scientific Research Innovation Foundation in Harbin Institute of Technology.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, K., Yan, Ps., Ding, Ql. et al. Diversity of culturable root-associated/endophytic bacteria and their chitinolytic and aflatoxin inhibition activity of peanut plant in China. World J Microbiol Biotechnol 29, 1–10 (2013). https://doi.org/10.1007/s11274-012-1135-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1135-x