Abstract
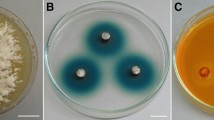
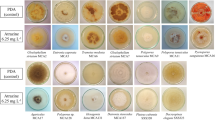
A survey to isolate native white rot basidiomycetes from Northeast Mexico was conducted in the forests of the Sierra Madre Oriental in the state of Nuevo León. A total of 92 isolates from at least 20 different genera, were screened on Bran-Flakes solid plate cultures for the production of ligninolytic oxidases and/or peroxidases with guaiacol and o-anisidine as substrates; their lignin depolymerizing potential using the polymeric dye Poly R 478; their ability to decolorize anthraquinonic (Remazol Brilliant Blue Reactive), azo (Acid Red 44) and triphenylmethane (Crystal Violet) dyes. Among all fungi tested, 15 isolates showed extensive decolorization of the three dyes within a week and gave a positive reaction in guaiacol and o-anisidine tests. Nine of them were also efficient degraders of Poly R-478. Two isolates (CS5 and CU1) showed decolorization of all dyes within 5 days, comparing favorably with reference strains of P. chrysosporium, Pleurotus ostreatus, and Bjerkandera adusta. Decolorization was associated with laccase activity in both isolates and reached 90% or more for all dyes within 24 h in 8-day-old liquid cultures. The coupling of pairs 2,4-dichlorophenol + 4-aminoantipyrine and 3-dimethylaminobenzoic acid + 3-methyl-2-benzothiazolinone hydrazone, strongly suggest that the laccases of both strains correspond to those considered of high redox potential. These strains are considered good candidates for bioremediation of dye polluted effluents due to their ligninolytic potential and decolorizing performance.




Similar content being viewed by others
References
Abadulla E, Tzanov T, Costa S, Robra K-H, Cavaco-Paulo A, Gübitz GM (2000) Decolourization and detoxification of textile dyes with a Laccase from Trametes hirsuta. Appl Environ Microbiol 66(8):3357–3362
Asgher M, Shah SAH, Ali M, Legge RL (2006) Decolourization of some reactive dyes by white rot fungi isolated in Pakistan. World J Microbiol Technol 22:89–93
Baughman GL, Weber EJ (1994) Transformation of dyes and related compounds in anoxic sediment: kinetics and products. Environ Sci Technol 28:267–276
Castillo MdP, Stenström J, Ander P (1994) Determination of manganese peroxidase activity with 3-methyl-2-benzothiazolinone hydrazone and 3-(dimethyamino) benzoic acid. Anal Biochem 218:399–404
Chung KT, Stevens SE Jr, Cerniglia CR (1992) The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol 18:175–190
De Jong E, de Vries FP, Field JA, van der Zwan RP, de Bont JAM (1992) Isolation and screening of basidiomycetes with high peroxidative activity. Mycol Res 96(12):1098–1104
Dhouib A, Hamza M, Zouari H, Mechichi T, Hmidi R, Labat M, Martínez MJ, Sayadi S (2005) Screening for ligninolytic enzyme production by diverse fungi from Tunisia. World J Microbiol Technol 21:1415–1423
Eggert C, Temp U, Eriksson K-EL (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62(4):1151–1158
Field JA, Jong E, Feijoo Costal G, Bont JA (1992) Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white rot fungi. Appl Environ Microbiol 58(7):2219–2226
Garfin DE (1990) One dimensional gel electrophoresis. In: Deutscher MP (ed) Methods in enzymology, vol 182. Guide to protein purification. Academic, New York, pp 425–44. 1ISBN 0-12-182083-1
Glenn JK, Gold MH (1983) Decolourization of several polymeric dyes by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol 45(6):1741–1747
Guzmán G (1980) Identificación de los hongos. Comestibles, venenosos y alucinantes. ed. Limusa, S.A. México, p 452. ISBN 968-18-0123-7
Hao OJ, Kim H, Chiang PC (2000) Decolourization of wastewater. Crit Rev Environ Sci Technol 30(4):449–505
Harkin JM, Larsen MJ, Obst JR (1974) Use of syringaldazine for detection of laccase in sporophores of wood rotting fungi. Mycologia 66:469–476
Heinfling A, Martínez MJ, Martínez AT, Bergbauer M, Szewzyk U (1998) Transformation of industrial dyes by manganese peroxidases from Bjerkandera adusta and Pleurotus eryngii in a Manganese-independent reaction. Appl Environ Microbiol 64(8):2788–2793
Heinzkill M, Bech L, Halkier T, Schneider P, Anke T (1998) Characterization of laccases and peroxidases from wood-rotting fungi (Family Coprinaceae). Appl Environ Microbiol 64(5):1601–1606
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microbiol Technol 30:454–466
INEGI (2001) XV Censo Industrial. Censos Económicos 1999. Industrias manufactureras subsector 32. Producción de textiles, prendas de vestir e industria del cuero. Productos y materias primas. ISBN 970-13-3334-9. INEGI, México, p 121
Jarosz-Wilkolazka AJ, Kochmanska-Rdest J, Malarczyk E, Wardas W, Leonowicz A (2002) Fungi and their ability to decolourize azo and anthraquinonic dyes. Enzyme Microbiol Technol 30:566–572
Jordaan J, Leukes WD (2003) Isolation of a thermostable laccase with DMAB and MBTH oxidative coupling activity from a mesophylic white rot fungus. Enzyme Microb Technol 33(2):212–219
Kiiskinen L-L, Rättö M, Kruus K (2004) Screening for novel-producing microbes. J Appl Microbiol 97:640–646
Knapp JS, Newby PS, Reece LP (1995) Decolorization of dyes by wood-rotting basidiomycete fungi. Enzyme Microb Technol 17:664–668
Maximo C, Pesoa MT, Costa-Ferreira M (2003) Biotransformation of industrial reactive azo dyes by Geotrichum sp CCMI 1019. Enzyme Microb Technol 32:145–151
McMullan G, Meehan C, Conneely A, Kirby N, Robinson T, Nigam P, Banat IM, Marchant R, Smyth WF (2001) Microbial decolourisation and degradation of textile dyes. Appl Microbiol Biotechnol 56:81–87
Mester T, Tien M (2000) Oxidation mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants. Int Biodeterior Biodegradation 46:51–59
Nerud F, Misurkova Z (1996) Distribution of ligninolytic enzymes in white rot fungi. Folia Microbiol 41:264–266
Ollikka O, Alhonmäki K, Leppären V-M, Glumoff T, Raijola T, Suominen I (1993) Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by lignin peroxidase isoenzymes from Phanerochaete chrysosporium. Appl Environ Microbiol 59:3605–3613
Paszczynski A, Crawford RL (1995) Potential for bioremediation of xenobiotic compounds by the white-rot fungus Phanerochaete chrysosporium. Biotechnol Prog 11:368–379
Pickard MA, Vandertol H, Roman R, Vazquez-Duhalt R (1999) High production of ligninolytic enzymes from white rot fungi in cereal bran liquid medium. Can J Microbiol 45:627–631
Podgornik H, Grgic I, Perdih A (1999) Decolorization rate of dyes using lignin peroxidases of Phanerochaete chrysosporium. Chemosphere 38:1353–1359
Pointing SB (2001) Feasibility of bioremediation by white –rot fungi. Appl Microbiol Biotechnol 57:20–33
Rabinovich ML, Bolobova AV, Vasil’chenko LG (2004) Fungal decomposition of natural aromatic structures and xenobiotics: a review. Appl Biochem Microbiol 40(1):1–17
Raghukumar C (2000) Fungi from marine habitats: an application in biodegradation. Mycol Res 104(10):1222–1226
Ramachandra M, Crawford DL, Hertel G (1988) Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl Environ Microbiol 52(12):3057–3063
Rodriguez E, Pickard MA, Vazquez-Duhalt R (1999) Industrial dye decolorization by laccases from ligninolytic fungi. Curr Microbiol 38:27–32
Saparrat MCN, Martínez MJ, Cabello MN, Arambarri AM (2002) Screening for ligninolytic enzymes in autochthonous fungal strains from Argentina isolated from different substrata. Rev Iberoam Micol 19:181–185
Stoscheck CM (1990) Quantitation of protein. In: Deutscher MP (ed) Methods in enzymology, vol 182. Guide to protein purification. Academic, New York, pp 62–63. ISBN 0-12-182083-1
Swamy J, Ramsay JA (1999) The evaluation of white rot fungi in the decoloration of textile dyes. Enzyme Microb Technol 24:130–137
Tekere M, Mswaka AY, Zvauya R, Read JS (2001) Growth, dye degradation and ligninolytic activity studies on Zimbabwean white rot fungi. Enzyme Microb Technol 28:420–426
Thorn RG, Reddy CA, Harris D, Paul EA (1996) Isolation of saprophytic basidiomycetes from soil. Appl Environ Microbiol 62(11):4288–4292
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol 161B:238–248
Walter M, Guthrie JM, Sivakumaran S, Parker E, Slade A, McNaughton D, Boyd Wilson KSH (2003) Screening for New Zealand native white-rot isolates for PCP degradation. Bioremediation J 7(2):119–128
Wariishi H, Valli K, Gold MH (1992) Manganese (II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. J Biol Chem 2676:23688–23695
Zhang F, Knapp JS, Tapley KN (1999) Development of bioreactor systems for decolourization of orange II using white rot fungus. Enzyme Microb Technol 24:48–53
Acknowledgments
The authors acknowledge the support received from the PAICYT, UANL under project number CA 822-04. G.G.S. and S.S.M. were recipients of CONACYT fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández-Luna, C.E., Gutiérrez-Soto, G. & Salcedo-Martínez, S.M. Screening for decolorizing basidiomycetes in Mexico. World J Microbiol Biotechnol 24, 465–473 (2008). https://doi.org/10.1007/s11274-007-9495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9495-3