Abstract
The efficiency of heterogeneous electro-Fenton technology on the degradation of recalcitrant organic pollutants in wastewater is glaringly obvious. This green technology can be effectively harnessed for addressing ever-increasing water-related challenges. Due to its outstanding performance, eco-friendliness, easy automation, and operability over a wide range of pH, it has garnered significant attention from different wastewater treatment research communities. This review paper briefly discusses the principal mechanism of the electro-Fenton process, the crucial properties of a highly efficient heterogeneous catalyst, the heterogeneous electro-Fenton system enabled with Fe-functionalized cathodic materials, and its essential operating parameters. Moreover, the authors comprehensively explored the major challenges that prevent the commercialization of the electro-Fenton process and propose future research pathways to countervail those disconcerting challenges. Synthesizing heterogeneous catalysts by application of advanced materials for maximizing their reusability and stability, the full realization of H2O2 activation mechanism, conduction of life-cycle assessment to explore environmental footprints and potential adverse effects of side-products, scale-up from lab-scale to industrial scale, and better reactor design, fabrication of electrodes with state-of-the-art technologies, using the electro-Fenton process for treatment of biological contaminants, application of different effective cells in the electro-Fenton process, hybridization of the electro-Fenton with other wastewater treatments technologies and full-scale analysis of economic costs are key recommendations which deserve considerable scholarly attention. Finally, it concludes that by implementing all the abovementioned gaps, the commercialization of electro-Fenton technology would be a realistic goal.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
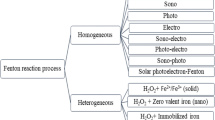
The extent of environmental contamination is heavily influenced by the magnitude of different industrial and anthropogenic activities. Different studies with different methods like electrochemical, biological, oxidation, adsorption, and coagulation have been performed to effectively decrease and remove different undesirable compounds in industrial wastewater. In the view of the current scenario, with a gradual reduction in water sources, the advanced oxidation processes (AOPs) have garnered substantial attention due to the ability to degrade organic pollutants into inorganic ions, CO2, H2O, or other harmless compounds (Shokri et al., 2020; Shokri and Sanavi Fard 2022a). The degradation of organic contaminants such as surfactants, pharmaceuticals, antibiotics phenolic compounds, dye molecules, herbicides, and pesticides is quite difficult (Ren et al., 2019; X. Zhao et al., 2016). Traditional water treatment processes are not always able to remove such emerging refractory pollutants. Their presence in water even at low concentrations and also their potential to generate detrimental compounds motivated the enhancement of AOPs (Shokri, 2017a, 2019; Karimi et al., 2020; Li et al., 2019a, 2019b; Mi et al., 2019a; Shokri and Sanavi Fard 2022b). Hence, the electro-Fenton has emerged as a green and cost-effective technology to produce remarkable quantities of hydroxyl radical (OH•) for the degradation of different recalcitrant organic compounds (Mousset et al., 2017; Shokri, 2017b; Zhang et al., 2020). Figure 1 presents the classification of electrochemical advanced oxidation processes (EAOPs) based on Fenton’s reaction chemistry utilized for the removal of organics from wastewater. Equation 1 shows the fundamentals of this process, where the oxidation of ferrous (Fe2+) to ferric (Fe3+) enhances the conversion of hydrogen peroxide (H2O2) to OH•. The constant generation of OH• needs both Fe2+ and H2O2. By cathodic reduction of Fe3+, the Fe2+ can be regenerated (Eq. 2). As can be seen from Eq. 3, by two-electron reduction of dissolved oxygen H2O2 is generated (Shokri et al., 2019).

Adapted from Brillas et al. (2009)
Classification of EAOPs based on Fenton’s reaction chemistry utilized for the removal of organics from wastewaters.
Nevertheless, despite easy operation and low-cost catalyst source, the classical homogeneous electro-Fenton has some prohibitive limitations. The formation of sludge, due to the presence of Fe2+ and Fe3+, causes precipitation of hydroxide which must be removed, increasing cost and hence decreasing overall performance. Because solid hydroxide species like Fe(OH)3 and Fe(OH)2 are soluble at pH values less than 4, as a result, harsh acidic condition is required to prevent their generation. Hence, the major challenge is that the solution pH should be acidified to stringent pH conditions around 2 to 3. To be more specific, the homogeneous electro-Fenton is effective only at limited ranges of pH (2.8–3.5). More importantly, this narrow range of working pH makes the neutralization of treated wastewater inevitable. Difficulty in the catalyst reusability and recyclability are also other drawbacks of this technology. In addition, providing pure oxygen is essential to generate hydroxide radicals (Iglesias et al., 2015; Mohadesi & Shokri, 2017, 2019).
Hence, to address the abovementioned challenges, heterogeneous electro-Fenton has advent as a promising technology (Hammouda et al., 2016; Tang et al., 2016). In contrast to conventional electro-Fenton, the heterogeneous electro-Fenton employs a solid catalyst and functionalized cathode for decomposing H2O2 to generate OH•. Hence, it enables easy separation and increases the feasibility of recycling the iron which is a crucial element in sustainable system operation. Several investigations have demonstrated that functionalized cathodic materials like graphite-felt, iron-modified carbon felt, Fe-Cu carbon aerogel, and ferrite can be many times (up to 10) used without a remarkable decrease in the process performance (Eda & Chhowalla, 2010; Huong Le et al. 2017; Nag et al., 2018). As a great advantage, this process can be operated over an extensive range of pH which obviates the requirement for pH adjustment and neutralization of the final effluent. In other words, it is a pH-independent process. This feature is crucial because real effluents or wastewaters have a broad range of pH and also are multi-component. In conclusion, the removal of sludge generation and additional post-treatment, reusability and recyclability of catalyst, decreasing energy consumption, without needing storage and transportation of highly unstable and dangerous H2O2 oxidizer are some of the major benefits of this process (Casado, 2019; Chang et al., 2020; Ganiyu et al., 2018).
Even though hetero-electro-Fenton can be utilized for an extensive range of pH, still the slow reduction of Fe2+ to Fe3+ in the Fe-based catalysts and long-run catalyst stability is challenging. Moreover, to supply effective degrees of OH•, significant and continuous concentrations of H2O2 are needed which can be problematic because the two-electron oxygen reduction reaction usually becomes more complex by the competing four-electron transfer reaction. As a result, significant attempts have been dedicated to the design and producing cathodic materials that accelerate the generation of greater dosages of H2O2. For the generation of H2O2, metals like Ru, Pd, Pt, and Au (Kim et al., 2014; Waldt et al., 2020) are effective catalysts, with proper conductivity and lower overpotentials. Nevertheless, the application of these metals is not completely economically feasible. As a result, carbon-dependent electrodes especially graphene are constantly developing to be utilized in electro-Fenton systems. Moreover, the selection of catalysts and materials for minimizing their adverse environmental effects and the feasibility of increasing the overall cost for the generation of solid catalysts are some of the common challenges which must be properly addressed in the heterogeneous electro-Fenton system.
So far, numerous experimental works have been conducted on the electro-Fenton process and there are several review papers that comprehensively explored different aspects of electro-Fenton-enabled systems (Brillas & Garcia-Segura, 2020; Casado, 2019; Ganiyu et al., 2018; Marlina & Purwanto, 2019; Meijide et al., 2021; Poza-Nogueiras et al., 2018; Wang et al., 2022; Yu & Breslin, 2020). However, to our best knowledge, there is not any unique paper with a specific focus on the challenges and future research directions of this process. In other words, it has attracted scant attention and is only limited to insufficient discussion in the final part of some papers. As a result, this paper thoroughly discussed major electro-Fenton challenges and pinpoints its future prospects to bridge this technical gap, besides motivating nonstop development of this green technology.
2 Principal Mechanism of Heterogeneous Electro-Fenton
Figure 2 demonstrates the schematic of both the heterogeneous and homogenous electro-Fenton reaction. In the heterogeneous reaction, Fe2+ is produced from the sacrificial anode and reacts with H2O2 in the bulk solution. In this case, to facilitate the reaction, iron salt can be added to the cell. In contrast, in the heterogeneous electro-Fenton process, it is desirable to maintain the Fe2+/Fe3+ couple in the solid state, and these couple reactions can be sustained if the cathode accelerates a two-electron reduction reaction to generate H2O2 (Ganiyu et al., 2018; Yu & Breslin, 2020).

Adapted from Yu and Breslin (2020)
Schematic of a heterogeneous and b homogeneous electro-Fenton process.
The decomposition of H2O2 by solid iron oxides, transition, or iron metals typically includes two conditions based on the solution pH (Ganiyu et al., 2018; Pham et al., 2009). At pH values less than 4.5, the system is governed by the redox cycling of dissolved Fe2+ and Fe3+. The considerable amounts of iron ions leach from iron-functionalized cathodic materials or the solid catalyst in an acidic environment releasing dissolved Fe2+ and Fe3+. Other transition metals redox couples like Co3+/Co2+ and Cu2+/Cu+ by leaching from solid catalyst into solution media can catalyze the decomposition of H2O2 to OH• (Ganiyu et al., 2017, 2018; Huang et al., 2018). Also, the presence of other transition metals besides iron in the solution might be promoting the reduction of Fe3+ to Fe2+ along with the catalytical decomposition of the H2O2, hence improving the performance of the electrolytic process (Zhong et al., 2013). Simultaneously, in the low acidic environment, the surface alkalescent \(\equiv {\mathrm{Fe}}^{\mathrm{III}}/{\mathrm{Fe}}^{\mathrm{II}}-\mathrm{OH}\) which occurred at the surface of the solid catalyst results in H2O2 decomposition. As a result, the dominant catalytic mechanism is based on both the solubility of the solid catalyst at lower pH values and the working pH (Ganiyu et al., 2018; Hammouda et al., 2016). Solid catalysts like natural iron minerals (Barhoumi et al. 2015, 2017a, 2017b) and synthetic nanoparticles of Fe and Fe-supported catalysts (Cruz et al., 2017; Garcia Rodriguez & Carrascal Domínguez, 2017) at the strongly acidic environment (pH lower than 3 which is optimal) undergo further leaching; hence, H2O2 decomposition is mainly occurred by the mechanism of homogeneous Fe3+/Fe2+. The homogeneous redox couples based on the applied cathodic material might be constantly reproduced at the cathode (Eq. 2) (Huong Le et al. 2017; Oturan et al., 2017). For several iron/transition metals including functionalized electrodes like Fe@Fe2O3/ACF, Fe, and Fe-Cu allophane nanoclays, Fe-Carbon-felt, and Fe-bentonite particle electrode similar mechanism has been reported, which can catalyze the decomposition of H2O2 in the bulk with the lowest amount of surface decomposition of H2O2 (Ganiyu et al., 2018; Garrido-Ramírez et al., 2016; Sklari et al., 2015). It is worth mentioning that Fe2+ oxidation and other transition metal ions at the anode surface have limited feasibility. Functionalized cathodic materials especially layered double hydroxide modified carbon-aerogel and carbon-felt demonstrated adequate stability in an acidic environment with minimum iron ions leaching (Ganiyu et al., 2018; H. Zhao et al., 2017). Thereby, when aforesaid functionalized cathodic materials are used in heterogeneous electro-Fenton at lower degrees of acidic environments, surface catalyzed decomposition of H2O2 by FeIII/FeII is remarkable. In conclusion, under such conditions, the catalytic degradation of H2O2 to OH• is by surface FeIII/FeII redox couple and homogeneous Fe2+ and Fe3+; however, the dominant mechanism is ascertained by the properties of the applied solid catalyst. In contrast, in neutral and alkaline environments, because Fe3+ is insoluble the contribution of dissolved iron ions to the decomposing H2O2 is expected to be insignificant (Ganiyu et al., 2018; Pham et al., 2012).
It must be mentioned that although the kinetics of heterogeneous electro-Fenton is complicated, the most influential parameters are certainly the generation rate of OH• from H2O2, which is governed by the solid catalyst properties, H2O2 production, and pH value (Ganiyu et al., 2018; Hou et al., 2014; Munoz et al., 2015). Until now, there is no consensus on reaction mechanisms for the entire oxidation process, but it is a widely held idea that the main reaction mechanism for the heterogeneous electro-Fenton is the Haber–Weiss circle mechanism (Ganiyu et al., 2018). The process of surface-catalyzed generation of H2O2 to OH• is summarized by the following reactions (Eqs. 3–9) (Ganiyu et al. 2018; Meijide et al., 2021; Yang et al., 2020):
Today, metal sulfides such as ZnS, PbS, CoS2, Cr2S3, WS2, and MoS2 are extensively utilized as perfect co-catalysts to improve the generation rate of OH•. The unsaturated sulfur atoms on the metal sulfides surface can take protons to produce H2S and release metallic active sites with reductive properties. Hence, co-catalysts promote the constant generation of \(\equiv {\mathrm{Fe}}^{\mathrm{II}}-\mathrm{OH}\) and OH• (Eqs. 10–15) (Meijide et al., 2021; Tian et al., 2021):
3 Heterogeneous Catalysts
Limitations of mass transformations are regarded as one of the main disadvantages of these catalytic processes (Poza-Nogueiras et al., 2018). After diffusion developed into the catalyst particles (external or film diffusion) and pores (internal diffusion), the catalytic reaction occurs; as a result, investigating the innovative heterogeneous catalyst which is capable of markedly minimizing the limitations of mass transformations is challenging (Meijide et al., 2021). According to the literature, natural iron minerals like magnetite, goethite, and hematite can potentially decompose H2O2 to OH• (Barhoumi et al., 2017a; Poza-Nogueiras et al., 2018). The feasibility of pyrite as a heterogeneous iron catalyst for antibiotic degradation was evaluated by Barhoumi et al. (2017a) who reported that in optimal conditions, after 8 h treatment, approximately 96% degradation was obtained. This work demonstrates the ability of pyrite for application in the electro-Fenton system as an effective solid catalyst. Based on Eqs. 16–18, the efficiency of the pyrite-dependent electro-Fenton was due to the self-regulator of iron concentration and pH in the system (Meijide et al., 2021):
Moreover, Droguett et al. (2020) have assessed the application of chalcopyrite for the mineralization of cephalexin which has a similar catalytic behavior as pyrite and performs like a self-regulator (Eq. 19):
For the removal of pharmaceuticals using heterogeneous electro-Fenton, natural iron-dependent composites have been explored (Kumar et al., 2019). Because of the significant reactivity of iron nanoparticles in the water, they present remarkable rates of passivation, oxidation, and surface energy. As a result, transition metals like copper have been integrated with them to develop their structure and overcome the abovementioned disadvantages. Due to this synergistic effect which drives by the physiochemical properties of each metallic material, the stability and degradation improved and made electrons transformation effective. The removal of nafcillin from an aqueous environment by bimetallic nanoparticles besides Fe and Cu monometallic was investigated by Campos et al. (2020). The reason for the application of bimetallic nanoparticles catalyst was to overcome the restrictions related to the application of ferromagnetic materials. In comparison with monometallic nanoparticles, the application of bimetallic nanoparticles facilitates the removal of the nafcillin with a lower treatment time. Nevertheless, in the case of catalyst reuse, the rate of contaminant removal is reduced to 77% because of the generation of CuFe2O4, Fe2O3, and FeO oxides on the surface of the nanoparticle. To explore the catalytic activity of prepared nano-layered double hydroxide including Cu and Fe on the degradation of gentamicin, Ghasemi et al. (2019) reported similar findings. Under optimum conditions, due to their hydrotalcite structure, not only ion exchange and surface area were enhanced but also low metal leaching following approximately complete antibiotic degradation was observed.
Regardless of iron-dependent catalysts, as discussed, under extensive ranges of pH, copper has similar redox properties. Due to the crucial effect of structure, different copper-driven perovskites have been explored. For instance, under circumneutral pH and moderate conditions, by application of paracetamol acting as the chemical probe, LaCu1-x TixO3, and LaCu1-x MnxO3 were synthesized and evaluated by Carrasco-Diaz et al. (2016). Due to the adequate activity of Ti and Mn in the Fenton-like processes, they were selected as the B-position metals. The findings demonstrated degradation of antibiotics was not due to the presence of Ti and Mn and Cu presented more redox activity. Moreover, significant metal stability of structure and little metal leaching proved the application of copper as a competitive and effective alternative catalytic material (Meijide et al., 2021).
New composites including iron nanoparticles have been prepared (Özcan et al., 2017). For example, as a competitive alternative for degrading enoxacin from an aqueous solution, Özcan et al. (2017) suggested Fe2O3-modified kaolin. Because of the environmentally friendly structure, significant adsorption potential, and low cost, mainly as catalyst supports clays like perlite and kaolin have been extensively used (Puga et al., 2020). In addition, recently, in order to improve reusability, stability, and catalytic activity as immobilization substrates, carbonaceous materials have been applied. The integration of porous carbon as shell and transition metal nanoparticle as core (core–shell structured catalysts) not only improves catalytic behavior but also simultaneously decreases catalyst aggregation (Meijide et al., 2021). For instance, aiming to degrade sulfamethazine solutions, Du et al. (2020) prepared Fe/Fe3C@PC as core–shell structured materials and assessed their performance. In comparison with bare iron oxides because of the synergistic effect of PC and Fe/Fe3C, Fe/Fe3C@PC presented improved efficiency. This improved performance further highlights the role of nano-engineering science in the electro-Fenton reaction. Moreover, because of the simple preparation techniques of graphite, significant specific area, remarkable chemical and mechanical stability, and outstanding electrical and thermal conductivity of graphene and its by-products, for catalyst immobilization, it has been regarded as a proper supporting structure (Divyapriya & Nidheesh, 2020; Meijide et al., 2021).
Table 1 demonstrates the summary of the investigations reported concerning the heterogeneous electro-Fenton process.
3.1 Essential Properties for Effective Heterogeneous Catalyst
It is crucial to explore the most essential factors to rate various materials as the best catalyst for the decomposition of H2O2. The stability of the material under a wide range of operational conditions and the catalytic activity are among the most essential factors. This is because significant degrees of stability ensure the catalyst reusability and materials with excellent catalytic activity are less influenced by experimental variations like changing solution pH (Ganiyu et al., 2018; Hou et al., 2014; Navalon et al., 2010). However, because typically contaminant to catalyst ratio ascertains the catalyst reusability, reusability is not a good benchmark to evaluate catalyst stability (Banić et al., 2011). This indicates that for solid catalysts with significant stability, further concentrations would increase the feasibility of catalyst reusability in various successive runs, while for low dosages it may decrease catalyst reusability. Since further concentrations of materials can increase destroying of produced OH• by its adverse attack on the catalyst surface (especially for moderately stable materials), optimization of catalyst concentration is crucial (Ortiz de la Plata et al., 2010). The organic component of the wastewater, the volume of treated wastewater, catalyst properties, and activity considerably determine the optimal concentrations of the catalyst. Moreover, since the metal ions might be generated complexes with carboxylic acids, which are typically the ultimate products of the electrochemical oxidation of organic contaminants, washing catalyst is crucial in the alkaline environment. In addition, the support material on which the catalysts are loaded should be non-radical scavenger and photo-resistant to prevent support decomposition. Also, in the case of the selection of proper catalysts or supports, the degree of their toxicity and environmental effects must be precisely determined. Because iron-based catalysts or supports have few environmental adverse effects, as a result, they are regarded eco-friendly materials (Ganiyu et al., 2018). Nevertheless, the application and disposal of treated solution or residual catalyst of some transition metals like Co, Ni, V, Mn, and Cu as a solid catalyst in the hetero-electro-Fenton systems possibly have significant environmental adverse effects due to their toxic nature (Barros et al., 2016; Liang et al., 2017). Also, aiming to ensure catalyst efficiency, their characterization data and the preparation/synthesis methods must be evaluated. Easy fabrication routes and economic feasibility are also essential for the selection of catalysts and supports and even for their commercialization. Further, because the generation rate of H2O2 and the catalyst activity ascertain the performance of the hetero-electro-Fenton systems, as a result, the cathodic materials modulated by iron species or the substrate used to support the solid catalyst must be chemically and physically non-reactive against H2O2. Hence, previous experiments are essential to assess both the capability of different cathodic materials for H2O2 generation and its decomposition by the substrate (Ganiyu et al., 2018).
4 Heterogeneous Electro-Fenton with Fe-Functionalized Cathodic Materials
The application of nano-driven materials like 3D electrodes in the electro-Fenton system results in substantial improvement in conductivity and mass transformation (Mohammadi et al., 2018). Thanks to such novel electrochemically induced processes, obtaining many micro-electrolysis cells and the improved surface contact area is undeniable which significantly improved overall process efficiency. Lately, iron-functionalized cathodic materials (Fig. 3) have been introduced to apply in hetero-electro-Fenton systems for the mineralization of recalcitrant organic contaminants. To end this, the functionalized material acts as both an iron source and an electrode. Frequently, the catalysts are impregnated or loaded on materials like carbon aerogel, graphite felt, carbon-felt, and active carbon/fiber which possesses more capacity to produce H2O2. Although these systems demonstrate substantial efficiency in contaminant removal, several operating restrictions like the limited working range of pH and low current performance might act as an impediment to their rampant application in water treatment (Meijide et al., 2021). In order to remove nonsteroidal anti-inflammatory drugs utilizing graphite felt and Ti-PbO2 as the cathode and anode, Fe–Ni foam particles on 3D electrodes were investigated by Mohammadi et al. (Mohammadi et al., 2018) and reported a double increase in the removal rate. They reported the reason is the higher dosage of radical production since nickel-foam electrodes possibly cause activation of oxygen to generate single oxygen. In addition, to degrade tetracycline a cupper-doped Fe@Fe2O3 core–shell nanoparticle deposited on a Ni-dependent foam as the cathode was studied by Luo et al. (2020). Because of the reduction in active Fe0 sites, it was reported that increasing copper dosage causes a reduction in catalytic efficiency. Under optimum loadings of copper and iron, catalyst recyclability was enhanced during 6 consecutive runs with insignificant degrees of iron leaching.

Adapted from Meijide et al. (2021)
Mechanism of the catalytic activation on the heterogenous electro-Fenton process under iron-functionalized cathodic material.
In another work, as an electro-Fenton cathode, the electrocatalytic efficiency of mesoporous MnXCo3-XO4 nanoparticle-modified carbon felt was explored by Mi et al., (2019a, 2019b). With increasing redox reactivity and electron transformation, a greater rate of ciprofloxacin was degraded which was attributed to the enhancement of the availability of active sites and the synergistic effect of embedded Mn and Co metals in the mesoporous structure. By five consecutive cycles with a minimum rate of ion leaching, the reusability of the regulated cathode was assessed.
5 Influential Parameters on Heterogeneous Electro-Fenton
Applied current density, catalyst and contaminant dosages, electrolyte, temperature, oxygen supply, solution pH, electrode material, treatment time, initial pollutant concentration, and cell design are the major operational parameters that determine the efficiency of the electro-Fenton process. Between them, solution pH, current density, and catalyst dosage have garnered significant focus. Nevertheless, energy consumption and related factors to oxidation performance should be explored to evaluate the efficiency of the electro-Fenton system. Here, these essential factors are discussed in depth.
5.1 Applied Current Intensity
Applied current intensity has a significant effect on the generation rate of OH• (Barhoumi et al., 2017b). Based on Eq. 3, the applied current is a driving force for the reduction of O2 to generate H2O2 at the cathode. At low current intensities, electro-generated H2O2 occurs under regulated electron transformation conditions. Hence, an increase in the current density improves charge distribution using the cathode, increasing OH• dosage in the bulk solution which are very reactive species in charge for pollutant degradation. Moreover, the remarkable increase of currents increases iron ions regeneration, and again process performance increases. However, the further increase can enhance side reactions like H2O2 decomposition (Eq. 20) and a reduction of as-produced protons to H2 at the cathode (Eq. 21), Also, the discharge of O2 at the anode based on Eq. 22 would become prominent which reduces the process efficiency (Meijide et al., 2021; Monteil et al., 2019):
According to the studies of other researchers, an increase in pollutant degradation with an increase in current density can be related to the Fe2+ regeneration and an enhancement in anodic oxidation (Bassyouni et al., 2017). The higher current density in the reaction medium can increase the accessible electrons for Fe2+ regeneration (Eq. 3), and more hydrogen peroxides are generated (Eq. 2). This phenomenon can result in the production of more hydroxyl radicals on the active sites of the heterogeneous catalyst. From an operational and economic points of view, the quick ruin and frequent anode replacement are uncomplimentary. Thus, the very high current density will not be used in wastewater treatment.
5.2 Solution pH
In the degradation of organic contaminants using hetero-electro-Fenton, solution pH plays a pivotal role because of catalyst surface charge and dissolution properties (Wang et al., 2013). Application of the heterogeneous catalyst enables working in circumneutral and alkaline environments and due to the H2O2 decomposition into water and oxygen at pH > 4.5, the efficiency of pollutant degradation is slow (Droguett et al., 2020). The constant hydrolysis of catalyst ions results in pH variations which improve contaminant removal by oxidizing free radicals (Luo et al., 2020; Meijide et al., 2021).
In a hetero-electro-Fenton process, the optimum pH is a function of various parameters including the zero-point charge of the catalyst (pHZPC), the pKa and the type of contaminant (cationic or anionic). The catalytic activity differs in different pH values, due to the leaching of iron from the catalyst and the formation of sludge (Shen et al., 2016). Also, H2O2 is unstable in alkaline pH. The precipitation of Fe3+ ions increase with increase in pH, which decreases the generation of Fe2+ and subsequently decreases the process efficiency. The scavenging reaction of OH• by proton can reduce the oxidation ability of OH• (Adel Niaei & Rostamizadeh, 2021; Benzaquén et al., 2020). It should be noted that at higher pH values the production of OH• is lower, while proton salvation generates the hydroperoxonium ion from H2O2 (El-Ghenymy et al., 2012). At pH > pHZPC and pH < pHZPC, the presence of proton on the surface of the hydroxyl groups generates a negative and positive charges, respectively. So, the pollutants with negative structures favor to adsorb on the surface of the catalyst in positive states.
5.3 Electrode Type
One of the essential elements in the performance of the electro-Fenton is the selection of electrode type. An operative cathode substantial should have a high overvoltage for the evolution of H2, high conductivity and stability, and low catalytic activity for the degradation of hydrogen peroxide (Khataee & Hasanzadeh, 2018). In addition, stable anodes with high overpotential for the evolution of O2, and subsequent production of OH•, are also important for the improvement of the Electro Fenton process (Sopaj et al., 2016). As demonstrated in Eq. 23, electrodes with significant O2 overvoltage can increase the generation of OH•s (Marlina & Purwanto, 2019).
In comparison with other kinds of anodes, to degrade different kinds of contaminants platinum anodes have been used greatly. In high-corrosive environments, platinum has been used as an electrode with high conductivity in recent decades (Panizza & Cerisola, 2009).
The Pt with high stability and conductivity has been employed as an anode. But it has a robust interaction with OH• and can hinder the availability of OH• for contaminant degradation. Therefore, other materials including IrO2/RuO2 coated Ti, boron-doped diamond (BDD), etc. have been applied in the process. However, recently, the boron-doped diamond anode is extensively utilized in the electro-Fenton systems (Marlina & Purwanto, 2019; Nidheesh & Gandhimathi, 2012).
Several studies revealed that the energy consumption, pollutant degradation and current efficiency of BDD were higher than Pt (Nidheesh et al., 2019). Correspondingly, the configuration of the reactor contributes efficiently to the current effectiveness for the accumulation and production of H2O2.
5.4 Ferrous Ion Concentration
During the electro-Fenton system, the sustainability of iron ion dosage is crucial. Due to Fenton’s reaction (Eq. 24), the addition of Ferrous ions as a catalyst in the solution can increase the oxidation power of electro-generated H2O2.
The electro-catalysis reaction can be introduced as the following (Eq. 25)
At the cathode, the H2O2 is electro-generated continuously. Therefore, an increase in the dosage of Fe2+ can increase the generated OH• (Eq. 25), which is the main reactive species for the degradation of pollutants. Generally, the performance of the electro-Fenton is influenced by Fe2+ dosage since as an oxidizing agent it had a significant effect on the reduction of the big molecule-like dyes in wastewater (Marlina & Purwanto, 2019). By the addition of 0.5 mM Fe2+ for 420 min, the efficiency in degradation of antimicrobial sulfamethazine was achieved at 94% (El-Ghenymy et al., 2013). After increasing the Fe2+ concentration to 0.1 mM in 30 min, aspirin removal reached 100% (Yang et al., 2018a, 2018b).
Pollutant degradation is directly proportional to the catalyst concentration. But after reaching a stable condition, further increases in catalyst dosage have an adverse impact on the removal performance. This phenomenon can be attributed to the scavenging effect of OH• and excessive iron leaching into the solution (Eqs. 26 and 27) (Droguett et al., 2020; Ghasemi et al., 2019; Meijide et al., 2021):
Moreover, further concentrations of the catalyst increase agglomeration in the solution, reduce the catalyst’s active surface area, and diminish the degradation rate (Ghasemi et al., 2019).
5.5 Oxygen Flow Rate
The rate of oxygen sparging is a main parameter that limits electro-Fenton efficacy. A superior sparging of O2 can increase the amount of dissolved oxygen (DO) and mass transfer in the process, and consequently improve the generation of H2O2. Therefore, the regeneration of H2O2 in the electro-Fenton system is affected by oxygen concentration. As a result, it can be considered one of the crucial factors which can limit the performance of the process (Marlina & Purwanto, 2019; Pimentel et al., 2008).
Based on the exploration of Xia et al. (2019), the optimum dosage of H2O2 was at 0.21 L/min since exceeding the oxygen flow rate from 0.28 L/min, increasing bubbles cause a reduction of H2O2 generation.
It should be noted that the amount of the produced H2O2 does not enhance with O2 flow rates linearly. After saturation of oxygen, extra intensification in O2 sparging cannot increase the concentration of DO in the system and the produced H2O2 is stable. Actually, further profits from extra increase in the O2 sparging rate are restricted. Wang et al. (2010) reported that at a fixed current density of 3.2 mA/cm2 the increase in O2 sparging from 50 cm3/min to 250 cm3/min resulted in about 30% enhancement in the removal of COD.
5.6 Distance Between Electrodes
The distance between electrodes affects the efficacy of electro-Fenton process as it governs the energy consumption and potential of the system. A decrease in the electrode distance results in a higher current flow and a reduced resistance between electrodes. The passing time of contributing ions in electro-Fenton process enhances with an increase in the distance of electrodes, and leads to a higher electrolysis time and lower degradation performance (Verma et al., 2013). The Fe2+ can be electro-regenerated from Fe+3 on the cathode, which is controlled by either mass transfer of Fe3+ across the cathode-solution interface or electron transfer between Fe3+ and cathode. The mass transformation of Fe3+ to the surface of cathode was limited by longer distances between electrodes (Thor et al., 2022), and therefore hinders the regeneration catalyst and electro-Fenton efficacy. An increase in the distance of electrodes gives a substantial increase in energy costs (Zakeri et al., 2021).
In the electro-Fenton process, another influential parameter is the electrode gap. When there is a little gap between electrodes, energy consumption is reduced and COD removal is increased (Hu et al., 2018; Marlina & Purwanto, 2019). During the removal of phenol by the application of iron electrodes, electrode distance and process performance are proportional together and it was reported that process efficiency has reached 75% by the addition of activated carbon and at an optimal distance of 4 cm. In another study, for the wastewater containing reactive blue 19, the performance and optimum gap for removal of total organic carbon are reported at 74.3% and 3 cm, respectively (Zhou et al., 2019).
5.7 Electrolyte
Another influential element in the electro-Fenton system is the electrolytes. This is because it increases solution conductivity and facilitates electron transformation (Marlina & Purwanto, 2019). Na2SO4, MgSO4, KCl, and NaCl are some of the widely applied electrolytes for increasing solution conductivity.
Due to the Joule phenomenon, the improvement in treatment efficiency achieved by KCl and NaCl can be related to their strong movement by guaranteeing the good passage of the current and diminishing the fatalities of energy in heat. The presence of OH• and chlorine ions simultaneously increased the degradation of dye (Cui et al., 2021). With an increase in the concentration of electrolytes, the concentration of the present ions in the solution increased which is responsible for the passage of the electric current, whereas the electrical resistance decreased. According to Malakootian and Moridi (2017), in comparison with other electrolytes for the removal of dyes, NaCl is the best one with 80% performance at 15 min.
5.8 Temperature
The treatment performance of Fenton and associated processes is directly affected by process temperature. The temperature has a negative influence on the efficacy of heterogeneous electro Fenton process since the concentration of dissolved O2 is reduced and the H2O2 molecules are degraded at high temperatures (Adachi et al., 2022; Miao et al., 2020). In the study of the degradation of the azo dye cationic red X-GRL by the electro-Fenton process by an activated carbon fiber cathode, the optimum temperature was 60 °C where decoloration proportionally increased by increasing temperature and obtained performance of 93.46%. The reason is that with increasing process temperature, the color allowance will be increased (Lei et al., 2010; Marlina & Purwanto, 2019).
It should be noted that from an economic point of view performing the heterogeneous electro-Fenton process at ambient temperature had several benefits such as the high applicability and low energy demand. Finally, it can be concluded that extreme temperatures either high or low may have an adverse effect on the process performance and can be influenced by the mentioned parameters.
5.9 Hydrogen Peroxide Concentration
In electro-Fenton processes, the initial concentration of H2O2 plays a pivotal role, and this parameter increases the OH• concentration which is directly proportional to the rate of contaminant removal (Marlina & Purwanto, 2019). For example, treatments of the liquid organic fertilizer manufacturing wastewater through electrocoagulation and electro-Fenton processes utilizing iron electrodes were explored by Akyol et al. (2013). They reported that wastewater from the fertilizer industry with 435 mg/L organic total can be reserved to 84% by raising H2O2 concentration to 25 mm in 10 min. In another study, Gümüş and Akbal (2016) reported that as H2O2 concentration increases, the allowance for phenol has increased, which also applies to the allowance for COD.
Moreover, unused portions of H2O2 with reductive capacity can consume the COD test and therefore resulting in the overvaluation of COD values, and the amount of error is related to the concentration of H2O2. Also, the additional amount of H2O2 is toxic to many organisms and intensely reduces the total efficacy in those cases where the Fenton technique functions as a pretreatment to biological methods (Shokri, 2017b). An appropriate concentration of H2O2 needs to be determined to maximize the efficacy of an electro-Fenton process whereas diminishing related disadvantages.
5.10 Treatment Time
It was clear that, during electro-Fenton process, the oxygen is injected into the solution continuously and subsequently a constant H2O2 was produced. In addition, the Fe2+ ions were produced at the cathode through the electrochemical reduction of Fe3+ ions.
According to the studies of Shokri (2017b), the efficiency of the electro-Fenton process for the removal of toluene was increased from 42.3 to 94.5%, with an increase in electrolysis time from 30 to 60 min and then it was increased narrowly due to the achievement of a saturation level after 90 min. The reason can be originated from the reduction in approachability of ferrous ions due to some competing reactions which can be approved by a dark brown color of solution established for the production of iron-organic complexes.
5.11 Initial Pollutant Concentration
In the wastewater treatment by all branches of AOPs, the pollutant degradation was decreased with an increase in initial pollutant concentration. This phenomenon can be explained by the competitive OH• consumption by the produced intermediates at the high initial concentration of contaminants. In high concentration of pollutants, extra treatment time is needed and subsequently the consumption of energy and the process costs are increased noticeably. Thus, it is reasonable to use electro-Fenton process for degradation of low dosages of pollutants and these findings agree with the results of Shokri (2017c).
However, for wastewater treatment at a large industrial scale, the dilution makes a large quantity of wastewater and enhances the treatment costs considerably. Therefore, the contaminants need to be diluted properly to balance the costs and profits for cost-efficient degradation of contaminants in electro-Fenton schemes.
6 Economic Analysis
It goes without saying that cost is considered one of the essential parameters in any industry. Concerning the application of the most effective strategy in the electro-Fenton systems, an exhaustive evaluation of their cost and efficiency is vital. To advance EAOPs against traditional wastewater treatment methods for application in a wide range, restrictions concerning operating cost, efficiency, and design must be overcome. In general, operational cost consists of different important elements like fixed costs, sludge dewatering and disposal, labor cost, system maintenance, electrodes, and chemicals application, and energy consumption (Ismail et al., 2021; Orkun & Kuleyin, 2012; Suhan et al., 2020). Notwithstanding the different cost-incurring parameters, close attention concerning economic assessment must be devoted to energy consumption which demonstrates the biggest share of the overall operating cost of the electro-Fenton system (Ghanbari & Moradi, 2015).
The quantity of chemical consumption, electrode material consumption, electrical energy consumption, and other costs such as maintenance cost, labor cost, etc. are all considered parts of the total operating cost. In the literature, it was measured based on the costs of chemical materials, electrode material ($ kg−1), and electrical energy ($ kWh−1). The operating cost was measured by Eq. 28 in terms of the removal of one kg COD per one cubic meter of synthetic wastewater (Bayramoglu et al., 2007; Kobya et al., 2011; Suhan et al., 2020; Varank et al., 2018).
where CC is the chemical consumption (kg.m−3 kg−1 COD removal), the coefficient a is the cost of chemicals ($kg−1), EMC is the electrode material consumption (kg.m−3 kg−1 COD removal), coefficient b is the cost of electrode material ($kg−1), EEC is the electrical energy consumption (kWh.m−3 kg−1 COD removal), and coefficient c is the cost of electrical energy ($kWh−1) (Suhan et al., 2020). The electrical energy consumption and the electrode material consumption were measured by Eqs. 29 and 30:
where t is the reaction time (h), I is the applied current (A), U is the applied voltage (V), X is the fraction elimination for the remediation time, V is the volume of wastewater (m3) and CMO is the initial contaminant concentration (kg.m−3), Mw is the molecular mass of the electrode (kg.mol−1), F is the Faraday’s constant (96,487 C.mol−1) and z is the number of electrons transferred (ZFe = 3) (Suhan et al., 2020).
Other relationships can be written in other forms as well. For an electrolyzed solution the mineralization current efficiency can be measured using Eq. 31 (Garcia-Segura & Brillas, 2011; Meijide et al., 2021):
where I is the applied current (A), t is the time (h), F is Faraday’s constant (96,500 C/mol−1), m is the number of carbon atoms of each molecule, V is the volume (L), n is the number of consumed electrons, C is the total organic carbon (TOC) removal (mg/L−1) and \(4.32\times {10}^{7}\) is the conversion factor (Meijide et al., 2021).
One of the major disadvantages of the electro-Fenton system is the feasibility of the production of higher amounts of toxic organic compounds in comparison with the parent contaminant. Hence, for analyzing the ratio of biodegradation of organic compounds, generally, carbon oxidation state (COS) and average oxidation state (AOS) are utilized (Eqs. 32–33) (Meijide et al., 2018; Vilar et al., 2012):
where COD is the chemical oxygen demand (mmol O2/L) at time t, TOC0 is the initial dosage of total organic carbon (mmol C/L), and TOC is its value at time t. The carbon oxidation state indicates the carbon oxidation performance based on the removed carbon from the solution as gaseous carbon dioxide. Based on the average oxidation state, the maximal value + 4 is associated with the maximum oxidized state of carbon (carbon dioxide), and the minimal value − 4 is associated with the most reduced state of carbon (methane) (Meijide et al., 2021).
In a comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton, and peroxi-coagulation methods for the treatment of textile wastewater conducted by Ghanbari and Moradi (2015), the maximum energy consumption is associated with electro-Fenton whereas it has the minimum iron consumption since Fe2+ in the electro-Fenton process is not deactivated by precipitation in the system. Besides high performance, electrochemical Fenton and electrocoagulation systems are appropriate from an economic standpoint because they need lower time and electrical current compared with the other ones. The significant energy consumption in peroxi-coagulation and electro-Fenton processes can be related to cathodic and anodic reactions in the solution for in situ electrically generated Fenton reagents (Ghanbari & Moradi, 2015).
In conclusion, exploring the economic assessment of electro-Fenton systems is crucial because designing low-cost, effective, and simple system still is a significant bottleneck for researchers (Turkay et al., 2017). Despite strong arguments from a technical and environmental viewpoint, still, a compelling argument from an economic standpoint is mandatory. Without persuasive arguments from economic, environmental, and technical standpoints, the application of the electro-Fenton process on an industrial scale will be snail-pacing (Ismail et al., 2021).
7 Challenges and Future Research Directions
In the past recent decades, extensive research attempts have been committed to the development of EAOPs, especially the electro-Fenton-enabled systems. For the rapid and sustainable generation of H2O2 and OH•, developing effective and low-cost cathodic materials is of prime concern. Despite outstanding achievements in the enhancement of electro-Fenton reactions, still, intensive research effort is required for reaching significant breakthroughs. Following are discussed some of the major challenges encountered during the operation of electro-Fenton-enabled systems.
Regardless of the low-cost cathodic generation of H2O2, problems associated with functionalized cathodic materials and supported catalysts are among the most challenging issues in the hetero-electro-Fenton system (Yang et al., 2017; Zhou et al., 2018). Significant catalyst leaching/dissolution in extremely acidic environments and catalyst fall-off from the support because of the mechanical wearing and long operations are two major challenges that have a significant adverse effect on the catalyst performance reusability and stability. Apart from limited cases such as iron-modified carbonaceous cathodes, rolling fabricated with gas diffusion electrodes and functionalized carbon aerogels, typically at extreme acidic conditions extra leaching or dissolution were detected, hence homogeneous catalyst mechanism by recycling Fe3+ and Fe2+ redox couple controlling the all-electrolysis process. In these conditions, since with increasing pH value, the involvement of homogeneous electro-Fenton reduces hence, the efficiency of the electrochemical system remarkably diminished. Moreover, irrespective of working pH values, with increasing the electrolysis time, the catalyst falls off from the cathode or support, and the mechanical wearing might be increased because the solution undermines the cohesive bond between the support and catalyst (Ganiyu et al., 2018). For instance, during the heterogeneous electro-Fenton treatment of AO7 at pH value 5.83 by solvothermal synthesized CoFe-LDH/CF cathode, because of the extreme solution agitation and hence undermining the adhesive bond between the CoFe-LDH and the CF, after 6 periods of 2 h repeated reuse, mechanical fall off was detected (Ganiyu et al., 2017). For iron sludge, activated carbon fiber/felt, and iron-loaded clay materials, similar results can occur. Nevertheless, since the catalyst (copper/cobalt or iron/iron compounds) is firmly entrenched in the bulk of carbon aerogels and becomes the basic part of its three-dimension network, the aforesaid challenge is narrowed down or even totally removed in functionalized carbon aerogels (Ganiyu et al., 2018).
Another significant challenge that is generally faced during the condition of the heterogeneous electro-Fenton process is catalyst agglomeration, especially in the case of unsupported catalysts. Nearly all synthetic unsupported heterogeneous catalysts like pure Fe°, Fe3O4, or Fe2O3 or their modified and doped are typically synthesized and utilized as nanoparticles, as a result typically with the passing of time and tendency toward the agglomeration of such particles is inevitable, which decreases the catalyst active site, efficient surface area, and overall process efficiency. This phenomenon is evident in the case of magnetic iron-containing nanocatalysts including Fe° and Fe3O4 where the agitation of the electrolytic system is substantially difficult and the nanoparticles stick to each other, although this system is advantageous for easy separation of the catalyst for reusability purposes. In addition, most synthetics supported or unsupported catalysts and iron-functionalized cathodic materials need multi-stages or complicated synthesizing methods which have restricted most exploration of the heterogeneous electro-Fenton process to lab-scale or pre-pilot scale. For instance, in the case of functionalized carbon aerogels their synthesis contains at least four various unrelated stages, hence making the synthesis route a little complicated, although based on the literature, functionalized carbon aerogels remain the most effective heterogeneous cathodic material or catalyst (Ganiyu et al., 2018).
To further enhance the potential of different electro-Fenton systems and satisfy future stringent standards of real wastewater treatment, the following recommendations concerning heterogeneous electro-Fenton systems are the salient points that still merit intensive research attention and must be taken into the limelight to reach a full-fledged technology.
7.1 Synthesizing Heterogeneous Catalysts Using Advanced Materials for Maximizing Recyclability and Stability
To fabricate heterogeneous cathodic materials, the raw materials are quite cost-prohibitive and unstable, consequently remarkably influencing their reusability in the electro-Fenton reaction. As a result, large-scale explorations of heterogeneous electro-Fenton reaction have been markedly limited. In order to reach viable operations, especially in developing areas, decreasing costs by modification of carbon-based materials besides providing sustainable catalyst reusability is crucial. In this context, intensive research on producing catalysts with lower environmental toxicity and significant catalytic activity and stability is crucial to making sure further advancements in heterogeneous electro-Fenton systems. Moreover, with respect to economic feasibility, cost-effective catalyst synthesis is essential to facilitate the application of heterogeneous electro-Fenton systems on an industrial scale. Hence, catalyst synthesizing methods with environmental and economic viability are one of the major problems which urgently demand more intensive research efforts in the wastewater treatment field. This challenge can be overcome by the focus on the fabrication of low-cost cathodic materials and catalysts with higher stability in harsh acidic conditions and remarkable activity in real wastewater. Because of the enhanced operational performance and eco-friendly nature of biomass-motivated carbon-based materials and nonmetal electro-Fenton catalysts, a comprehensive investigation of their application must be conducted. For minimizing the cost of the electro-Fenton reaction, efficient anodic materials except for noble metals and boron-doped diamonds must be explored and produced. A clear insight into the potential pH range of the reaction will be advantageous for the fabrication of cost-effective and efficacious electrodes for electro-Fenton (Ismail et al., 2021).
Also, future research must be tailored toward the cost and environmental effects of both available and forthcoming functionalized cathode and solid catalysts. Previous research on the heterogeneous electro-Fenton process has not been successful to mitigate these crucial challenges, especially the environmental effect of the nano catalysts on the biotic component of the ecosystem of water reservoirs, where wastewater is discharged after treatment. This data is essential for both policymakers and the research community (Ganiyu et al., 2018).
Moreover, the restricted Fenton activity is a significant challenge faced in heterogeneous electro-Fenton systems. Because of the special electronic characteristics of the single metal active site, to achieve the highest atomic performance, recently the atomically distributed active metal center has become an innovative research area. Nevertheless, still limited explorations on single-atom catalysts as heterogeneous Fenton catalysts are performed; hence, it is valuable for exploring the ways to enhance the catalytic activity for degrading organic contaminants (Wang et al., 2022).
7.2 Full Realization of Activation Mechanisms of Hydrogen Peroxide
The activation mechanisms of H2O2 to OH• have not been completely explored and still, there is no unanimous agreement on the contributed reactions in the literature. As a result, more intensive explorations are needed to realize the mechanisms and reactions that affect hetero-catalytic degradation of the H2O2. For this purpose, in situ spectroscopic methods like DFT, EIS, and ATR-FTIR and microscopy investigation considering the treated solution might be a valuable tool in realizing the mechanisms and reactions integrated into H2O2 activation, hence providing appropriate design of the electrochemical reactors and optimizing the operating conditions (Ganiyu et al., 2018).
Also, the performance of electro-Fenton systems greatly relies on the synthesizing of H2O2. Nevertheless, still, enhancement of electro-generated H2O2 is problematic due to the lower H2O2 performance and inadequate Faradaic efficiency primarily arising from the catalytic activity and selectivity. Only limited studies concerning viable instruments for H2O2 generation and water treatment are available and all of them have low performances and complicated designs. Hence, enhancing catalyst and reactor designs is crucial for their commercialization. In addition, the scavenging effect of OH• by natural organic compounds and inorganic ions in real-world wastewater causes significant energy consumption and insufficient decrease of targeted contaminants. Moreover, OH•-induced heterogeneous electro-Fenton systems cannot selectively remove the refractory organic contaminants. Hence, the selective removal of intended contaminants with substantial toxicity and insignificant dosages during the treatment of real wastewater is highly problematic, which will inevitably widen the application range of hetero-electro-Fenton systems (Wang et al., 2022).
7.3 Detailed Investigation on Treatment of Biological Pollutants
Most available studies on the electro-Fenton method are only focused on the degradation of the different types of organic contaminants and limited studies are performed on the removal of viruses and microbes. In the middle of worldwide pandemic outbreaks and the advent of different contagious diseases, developing the application of the electro-Fenton process for microbial disinfection is absolutely crucial. The application of the photo-electro-Fenton process can enhance the performance of electro-Fenton treatment. To end this, by UV active inorganic nanoparticles powerful free radicals can be generated to destroy microbes and even coronavirus because it can perform as an effective anti-viral coating. The nanostructures can trap the virus and initiate chemical reactions using ultraviolet sources. Moreover, to decrease the cost of UV lamps, direct sunlight can be harnessed and perform as a visible light source for photo-electro-Fenton. Besides, the integration of different wastewater methods like electrocoagulation with electro-Fenton reaction can further advance decontamination performance (Nair et al., 2021).
7.4 Comprehensive Analysis of Economic Cost
The economic cost is the strong limitative parameter for large-scale applications of electro-Fenton systems. To obviate this limitation, compared to already available traditional wastewater treatment methods, electro-Fenton process must be quite cost-competitive. Economic costs could be measured in terms of outlay and operating costs. The overall process cost can fluctuate extensively because each kind of electro-Fenton system possesses various reactor designs and optimal operating factors. Techno-economic analysis of electro-Fenton systems demonstrates that in comparison with the electrical costs, the highly efficient anodic materials and the raw materials for heterogeneous catalysts have the highest share of the overall costs. Explorations on the real wastewater and methods of selective contaminant treatment must be performed to achieve a precise cost evaluation. However, according to the bibliometric results of Ismail et al. (2021), the economic analysis of electro-Fenton systems is extremely restricted and usually overlooked. Most of the research papers only evaluated energy consumption parameters and unfortunately, it is only limited to reactor cell analysis; hence, limited research works explored the calculation of electrode and chemicals consumption comprehensively. However, for effective evaluation of the economic feasibility of the process, only these data are not adequate. Thus, future research must be intensively focused on the economic cost of the electro-Fenton process, concerning reactor, electrode, energy, and chemical outlay costs (Ismail et al., 2021).
In addition, biomass-based carbon materials have garnered significant attention in oxygen reduction reactions. Different studies have mitigated the energy consumption challenge in the electro-Fenton process using triboelectric nanogenerators by which conversion of the mechanical energy to electrical energy occurred, as a result offering a self-enabled electro-Fenton system. However, they still need further research work to be mature enough for application in industrial-scale processes (Nair et al., 2021).
7.5 Combination of Electro-Fenton with Other Wastewater Treatment Systems
Conduction of the electro-Fenton system as a single unit for any wastewater treatment process due to its operational fundamentals is relatively challenging. Hence, for complete exploitation of electro-Fenton potential, it could be integrated with other wastewater treatment systems to design a hybridized process. Previous researchers have designed various incorporated methods including electro-Fenton integrated with membrane filtration system (Yang et al., 2019), integration of electro-Fenton with AOPs (Oriol et al., 2019), electro-Fenton as pretreatment to biological methods (Changotra et al., 2019; Popat et al., 2019), and electro-Fenton-driven systems integrated with electrochemical methods like electro-oxidation and electrocoagulation (Nordin et al., 2019; Zazou et al., 2019). According to the findings of past investigations, combined methods due to the feasibility of offering lower operating costs present outstanding overall efficiency, especially in terms of energy consumption in comparison with individual electro-Fenton treatment units. As a result, the economic feasibility of the system for industrialization will increase along with a highly effective and optimized coupled wastewater treatment system. The system’s capability for degradation of an extensive range of contaminants will increase with having at least two integrated methods; hence, an efficient and viable system can be established (Ismail et al., 2021). All in all, since integrated electro-Fenton systems have demonstrated promising results regarding overall efficiency and operational cost, the investigation of design and optimization of coupled systems could be comprehensively explored to fully exploit its potential.
7.6 Conduction of Life-Cycle Assessment to Explore Environmental Footprints and Potential Adverse Effects of Side-Products
For exploring the environmental effects of the electro-Fenton system, its life-cycle assessment is crucial. This assessment before system scale-up is essential because it can help prevention of any possible unfavorable repercussions derived from process development. Despite the outstanding performance of EAOPs for the degradation of extensive ranges of contaminants, toxicity evaluation of electro-Fenton effluents to minimize toxic side-products disposal, is extremely urgent. Based on the nature of the contaminant or wastewater under treatment, the different compounds in the solution will result in the generation of different dosages of toxic side products. Hence, assessment of the degree of side-product toxicity driven by the electro-Fenton treatment is quite essential because proper recovery procedures can be measured (Ismail et al., 2021).
The degraded organic contaminants may be degraded but not mineralized totally. The created byproducts may hold higher harmfulness than their initial parent pollutant; therefore, it is essential to clarify the noxiousness of alteration crops in the wastewater treatment methods. According to the studies of Khan et al. (2019), the Ecological Structure Activity Relationships (ECOSAR) program based on quantitative structure–activity relationship (QSAR) has been extensively used to assess the poisonousness of degradation intermediates. The three distinctive aquatic organisms such as daphnia, green algae, and fish are selected as model elements in ECOSAR program. Chronic and acute toxicities of organic contaminants and their conversion products are evaluated. The acute toxicity is stated by LC50 of fish and daphnia (concentrations of 50% fish and daphnia dye after 96 h and 48 h) and EC50 of algae (concentration of 50% green algae is damaged after 96 h). Moreover, other approaches have been used to detect the toxicity of intermediates including algae growth experience (Li et al., 2019a, 2019b), survival test of brine shrimps A. salina (Gong et al., 2020), activated sludge inhibition test (Cao et al., 2019), luminescent bacteria assay Vibrio fischeri (Deng et al., 2017), and Lactuca sativa seeds root length test (Zeng et al., 2020).
In other words, a clear realization of the degradation mechanism of different types of contaminants and the exploration of precise side products can be notably useful for real wastewater treatment. In comparison with real contaminants, different side-products can be potentially hyper-toxic. In-depth examinations of the possible effect of these side-products on the environment will be of great assistance for enhancement in real applications. Hence, in this way, any secondary contamination which possibly is generated because of the penetration and leaching will be decreased and curbed. Moreover, to avoid different negative impacts like environmental toxicity, the degree of carbon leakage must be regulated. For enhancing the side-products mineralization and reducing overall energy consumption and cost, the hybridization of electro-Fenton reactors with biological treatment could be one of the excellent methods. Moreover, investigation on sludge recyclability can be relatively attractive because typically electro-Fenton is a sludge-generating system. In this context, this sludge can be transferred to useful products like catalysts rather than disposed of. As a result, special attention to the careful exploration of life-cycle assessment, sludge control, and evaluation of the rate of side-products toxicity will conspicuously increase the opportunity for industrialized electro-Fenton-enabled systems (Nair et al., 2021).
7.7 Application of Different Effective Cells in Electro-Fenton Technology
In the electro-Fenton process, H2O2 is produced electrochemically at the cathode. An iron catalyst (Fe2+ or Fe3+) is added to the solution. In the electro-Fenton process, by injecting oxygen or air and reducing it in a suitable cathode, electrochemical production of oxygenated water is performed and in the presence of iron ions and by the Fenton reaction, OH•s are performed. Figure 4 shows a bench scale of discrete (a, b, c) and non-discrete (d, e, f) cells of electro-Fenton systems. In separate cells, the cathode and anode are in separate reactors and are connected by a salt bridge or any of the anode and cathode membranes, while in non-separate cells the cathode and anode are housed inside a reactor. The reference electrode in types (c) and (f) is of the saturated calomel electrode (SCE) type.
In discrete cells, the process was advanced by the spontaneous degradation of pollutants by reactive oxygen species (ROS) and mainly the produced OH• in the anode. In a non-discrete cell, the organic pollutants were degraded by OH• produced by the homogeneous Fenton method. The pollutant can be degraded by the OH• produced by the oxidation of water along with oxygen and an anode with high voltage (M) due to the following equation (Eq. 34).
Performing this process in its entirety is called paired electrocatalysis, because oxidizing agents can be formed in both anodic and cathodic reactions.
7.8 Upgrading Bench Laboratory Scale to Pilot Plant Scale and Better Reactor Design
Despite the fact that still more comprehensive studies are needed to fully improve this technology, the electro-Fenton system has shown its future viability and capability to be upgraded to a pilot plant scale for industrialization. Nevertheless, because still most of the researchers are focusing on laboratory-scale exploration, limited investigations have been performed on the pilot plant electro-Fenton. Because of the significant risk related to the feasibility of efficient operation of the system using electrodes with large dimensions and in the condition of remarkable volumes of effluent, conduction of a wide-range electro-Fenton system has been a significant challenge for the research community. Also, in spite of the significant benefits of the electro-Fenton reaction for the complete degradation of pollutants, implementing pilot-scale investigations of its treatment cost is not economically feasible. In other words, because of the fundamental shortcoming in pilot-scale studies, there is not enough data concerning energy consumption for pilot-scale design which further highlights the necessity of intensive research. In addition, in comparison with laboratory-scale designs, the treatment of real-world wastewater because of its complicated formulation is burdensome. As a result, with respect to the mineralization degree and overall process performance, during the electro-Fenton treatment process, they behave differently. Hence, considering limitative parameters like electrical resistance, mass transformation, etc. for designing pilot-scale reactors, intensive research efforts must be performed (Ismail et al., 2021).
Nevertheless, it is advisable that researchers must be eager against this issue to demonstrate the potential capability of electro-Fenton in the degradation of recalcitrant organic contaminants in wastewater at a large scale in comparison with traditional wastewater treatment methods. For the industrialization of electro-Fenton technology, firstly its scale-up is absolutely crucial because in this stage process viability in terms of the treatment of high-volume wastewaters with a significant concentration of contaminants and constant operability must be explored. The reason is that almost all of the results of the researchers performed on a batch laboratory scale are restricted to the synthetic contaminants with lower dosages or small volume wastewater which do not stand for real conditions. In addition, to ensure the effective operation of large-scale electro-Fenton systems in dealing with large volumes of real wastewater, reactor design is essential. Flow reactors that utilize heterogeneous electro-Fenton cathodes are still limited and have not been completely explored; hence, there are tremendous opportunities for better and full-scale applications of them (Nair et al., 2021). In other words, evaluations on flow reactors utilizing both functionalized cathodic materials and solid catalysts have received scant attention in the literature, which indicates that intensive research is required to explore the feasibility of the hetero-electro-Fenton system in flow reactors to alleviate the engineering and economic outlooks (Ganiyu et al., 2018). On the whole, remarkable research work is needed for the commercialization of the electro-Fenton reaction without jeopardizing its overall performance.
7.9 Electrode Fabrication and Development for Maximizing Process Performance
Both anodic and cathodic materials which are known as electrodes play a pivotal role in the determination of electro-Fenton systems. Especially, for wide-range applications of the electro-Fenton process, the nature of the electrode, efficiency, and lifetime are the most crucial parameters that will remarkably influence the overall cost of wastewater treatment. In general, 3D electrodes and porous carbon-dependent materials are desirable for cathode because they can enable O2 adsorption on the electrode surface for activation of O2 reduction to H2O2. By the application of different carbon and nanocarbon materials besides catalysts impregnation, several innovative modified carbon electrodes and carbon-PTFE gas diffusion electrodes have been synthesized by various researchers (Ridruejo et al., 2018; Yang et al., 2018a, 2018b; Ye et al., 2019). Graphene (carbon allotrope) and different transition metals are several cases of catalytic materials that have been explored (Ismail et al., 2021). However, almost all of the advancements on present carbon-based electrodes are operable only at the lab scale; as a result, scale-upping for application in real-world conditions is problematic. For industrialization of them, still, various factors including enhanced coating approaches for the prevention of nanoparticle agglomeration and efficient distribution of carbonaceous materials in solvents are required to be carefully optimized (Nair et al., 2021).
Regardless of cathodic materials, typically anodic materials are fabricated by metals or metal oxides which are chemically inert electrodes. In many types of research, in comparison with other ones boron-doped diamond has an excellent ability to perform as anodic material; however, it is cost-prohibitive and hence is not appropriate for application on an industrial scale. Actually, other valuable metallic anodes such as Pt and Ag are not so proper for wide-range applications. Hence, for the production of excellent and cost-competitive electrodes with significant electrode poisoning prevention, longer lifetime, and remarkable catalytic activity, comprehensive exploration is essential. Moreover, for modification of carbon-dependent cathode, since catalysts must present significant activity toward two oxygen reduction reactions at the low employed potentials, the selection of proper materials is crucial for catalyst synthesis on the cathode. From an economic outlook, optimization of catalyst costing and electrodes can be triggered using cutting-edge fabrication methods and mass production to minimize the preparation cost (Ismail et al., 2021).
Moreover, already, many research attempts have been performed for the development of practical cathodic materials for wastewater treatments using electro-Fenton reactions. For instance, although carbonaceous materials are observed to be highly competitive cathodes in electro-Fenton reactions, controlling and processing them needs cutting-edge technologies and careful examination. Almost all of the synthesis techniques entail complicated methods, which significantly require meticulous attention and some precautionary measures, as a result, increasing the overall cost of the process. Moreover, careful attention must be given to the development of cathodic materials that can be constructed from easily available and inexpensive materials like biochar-based carbon. Biomass-motivated carbon-based cathodic materials could provide significant surface area besides various heteroatoms that can present active sites for the enhancement of oxygen reduction reaction (Nair et al., 2021). Using chemical functionalization, for realizing the influence of various kinds of external atoms and their integrations on enhancing the quantity of H2O2 generation, still many mathematical and theoretical explorations are required to be implemented. Also, a basic realization of doping impacts on electrode–electrolyte interactions and various kinds of carbon-based materials still is insufficient (Nair et al., 2021).
Moreover, hetero atoms doping like nitrogen, fluorine, etc. with carbon-based materials improve charge distribution and present higher active sites for H2O2 generation. Heterogeneous electro-Fenton systems utilizing metal or metal oxide-modified cathodic materials can offer a better promise to investigate innovative materials for more efficient reactor designs. Numerous attempts have been devoted to iron-free reactions by developing a metal-free catalyst for activating H2O2 to OH•. Nevertheless, still, documentaries on designing low-cost wide-range cathode fabrication and long-term stable cathodes under real-world conditions are not available. Also, intensive research must be implemented on the kinetic and theoretical features of the electro-Fenton reaction for the fabrication of efficient cathodic materials for generations of H2O2 and contaminant mineralization (Nair et al., 2021).
8 Final Remarks
The heterogeneous electro-Fenton process has advent as one of the most interesting and efficient methods for wastewater treatment due to the catalyst reusability in several operations and its operability across extensive pH ranges. The environmentally friendly operation, reusability, easy separation, and significant performance and stability over an extensive pH window are some of the major hallmarks of the proper heterogeneous catalysts. However, there are several essential challenges associated with the heterogeneous electro-Fenton process for organic contaminants treatments including multi-stage/complicated methods for synthesizing functionalized cathodic materials or catalysts, catalyst agglomeration, catalyst dissolution in harsh acidic environments, and catalytic fall-off.
In summary, for reaching a mature and industrialized heterogeneous electro-Fenton process, the following are the available gaps that merit special attention:
-
(1)
Synthesizing heterogeneous catalysts using advanced materials for maximizing recyclability and stability
-
(2)
Full realization of activation mechanisms of H2O2
-
(3)
Detailed investigation on treatment of biological pollutants
-
(4)
Comprehensive analysis of economic cost
-
(5)
Combination of electro-Fenton with other wastewater treatment systems
-
(6)
Conduction of life-cycle assessment to explore environmental footprints and potential adverse effects of side-products
-
(7)
Application of different effective cells in the electro-Fenton process
-
(8)
Upgrading bench laboratory scale to pilot plant scale and better reactor design
-
(9)
Electrode fabrication and development for maximizing performance
Data Availability
All data analyzed or generated during this research are involved in this manuscript.
References
Adachi, A., El Ouadrhiri, F., Kara, M., El Manssouri, I., Assouguem, A., Almutairi, M. H., et al. (2022). Decolorization and degradation of methyl orange azo dye in aqueous solution by the electro fenton process: Application of optimization. Catalysts, 12(6), 665. https://doi.org/10.3390/catal12060665
Adel Niaei, H., & Rostamizadeh, M. (2021). Iron modified zeolite carrier for efficiently pharmaceutical pollutant degradation in heterogeneous electro-Fenton: Influence factors and kinetic. Environmental Progress and Sustainable Energy, 40(3), e13570. https://doi.org/10.1002/ep.13570
Akyol, A., Can, O. T., Demirbas, E., & Kobya, M. (2013). A comparative study of electrocoagulation and electro-Fenton for treatment of wastewater from liquid organic fertilizer plant. Separation and Purification Technology, 112, 11–19. https://doi.org/10.1016/j.seppur.2013.03.036
Banić, N., Abramović, B., Krstić, J., Šojić, D., Lončarević, D., Cherkezova-Zheleva, Z., & Guzsvány, V. (2011). Photodegradation of thiacloprid using Fe/TiO2 as a heterogeneous photo-Fenton catalyst. Applied Catalysis B: Environmental, 107(3–4), 363–371. https://doi.org/10.1016/j.apcatb.2011.07.037
Barhoumi, N., Labiadh, L., Oturan, M. A., Oturan, N., Gadri, A., Ammar, S., & Brillas, E. (2015). Electrochemical mineralization of the antibiotic levofloxacin by electro-Fenton-pyrite process. Chemosphere, 141, 250–257. https://doi.org/10.1016/j.chemosphere.2015.08.003
Barhoumi, N., Olvera-Vargas, H., Oturan, N., Huguenot, D., Gadri, A., Ammar, S., et al. (2017a). Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-Fenton process with solid catalyst chalcopyrite. Applied Catalysis b: Environmental, 209, 637–647. https://doi.org/10.1016/j.apcatb.2017.03.034
Barhoumi, N., Oturan, N., Ammar, S., Gadri, A., Oturan, M. A., & Brillas, E. (2017b). Enhanced degradation of the antibiotic tetracycline by heterogeneous electro-Fenton with pyrite catalysis. Environmental Chemistry Letters, 15(4), 689–693. https://doi.org/10.1007/s10311-017-0638-y
Barros, W. R. P., Steter, J. R., Lanza, M. R. V., & Tavares, A. C. (2016). Catalytic activity of Fe3-xCuxO4 (0≤x≤0.25) nanoparticles for the degradation of Amaranth food dye by heterogeneous electro-Fenton process. Applied Catalysis B: Environmental, 180, 434–441. https://doi.org/10.1016/j.apcatb.2015.06.048
Bassyouni, D. G., Hamad, H. A., El-Ashtoukhy, E. S. Z., Amin, N. K., & El-Latif, M. M. A. (2017). Comparative performance of anodic oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. Journal of Hazardous Materials, 335, 178–187. https://doi.org/10.1016/j.jhazmat.2017.04.045
Bayramoglu, M., Eyvaz, M., & Kobya, M. (2007). Treatment of the textile wastewater by electrocoagulation. Economical Evaluation. Chemical Engineering Journal, 128(2–3), 155–161. https://doi.org/10.1016/j.cej.2006.10.008
Benzaquén, T. B., Ochoa Rodriguez, P. A., Cánepa, A. L., Casuscelli, S. G., Elías, V. R., & Eimer, G. A. (2020). Heterogeneous Fenton reaction for the treatment of ACE in residual waters of pharmacological origin using Fe-SBA-15 nanocomposites. Molecular Catalysis, 481. https://doi.org/10.1016/j.mcat.2018.11.010
Brillas, E., & Garcia-Segura, S. (2020). Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Separation and Purification Technology, 237, 116337. https://doi.org/10.1016/j.seppur.2019.116337
Brillas, E., Sirés, I., & Oturan, M. A. (2009). Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chemical Reviews, 109(12), 6570–6631. https://doi.org/10.1021/cr900136g
Campos, S., Salazar, R., Arancibia-Miranda, N., Rubio, M. A., Aranda, M., García, A., et al. (2020). Nafcillin degradation by heterogeneous electro-Fenton process using Fe Cu and Fe/Cu nanoparticles. Chemosphere, 247, 125813. https://doi.org/10.1016/j.chemosphere.2020.125813
Cao, J., Lai, L., Lai, B., Yao, G., Chen, X., & Song, L. (2019). Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chemical Engineering Journal, 364, 45–56. https://doi.org/10.1016/j.cej.2019.01.113
Cao, P., Quan, X., Zhao, K., Chen, S., Yu, H., & Su, Y. (2020). High-efficiency electrocatalysis of molecular oxygen toward hydroxyl radicals enabled by an atomically dispersed iron catalyst. Environmental Science and Technology, 54(19), 12662–12672. https://doi.org/10.1021/acs.est.0c03614
Carrasco-Díaz, M. R., Castillejos-López, E., Cerpa-Naranjo, A., & Rojas-Cervantes, M. L. (2016). Efficient removal of paracetamol using LaCu1−xMxO3 (M = Mn, Ti) perovskites as heterogeneous Fenton-like catalysts. Chemical Engineering Journal, 304, 408–418. https://doi.org/10.1016/j.cej.2016.06.054
Casado, J. (2019). Towards industrial implementation of electro-Fenton and derived technologies for wastewater treatment: A review. Journal of Environmental Chemical Engineering, 7(1), 102823. https://doi.org/10.1016/j.jece.2018.102823
Chang, Y. M., Lin, H. W., Li, L. J., & Chen, H. Y. (2020). Two-dimensional materials as anodes for sodium-ion batteries. Materials Today Advances, 6, 100054. https://doi.org/10.1016/j.mtadv.2020.100054
Changotra, R., Rajput, H., & Dhir, A. (2019). Treatment of real pharmaceutical wastewater using combined approach of Fenton applications and aerobic biological treatment. Journal of Photochemistry and Photobiology a: Chemistry, 376, 175–184. https://doi.org/10.1016/j.jphotochem.2019.02.029
Cheng, S., Shen, C., Zheng, H., Liu, F., & Li, A. (2020). OCNTs encapsulating Fe-Co PBA as efficient chainmail-like electrocatalyst for enhanced heterogeneous electro-Fenton reaction. Applied Catalysis B: Environmental, 269, 118785. https://doi.org/10.1016/j.apcatb.2020.118785
Cruz, A., Couto, L., Esplugas, S., & Sans, C. (2017). Study of the contribution of homogeneous catalysis on heterogeneous Fe(III)/alginate mediated photo-Fenton process. Chemical Engineering Journal, 318, 272–280. https://doi.org/10.1016/j.cej.2016.09.014
Cui, T., Xiao, Z., Wang, Z., Liu, C., Song, Z., Wang, Y., et al. (2021). FeS2/carbon felt as an efficient electro-Fenton cathode for carbamazepine degradation and detoxification: In-depth discussion of reaction contribution and empirical kinetic model. Environmental Pollution, 282, 117023. https://doi.org/10.1016/j.envpol.2021.117023
Deng, J., Ge, Y., Tan, C., Wang, H., Li, Q., Zhou, S., & Zhang, K. (2017). Degradation of ciprofloxacin using Α-MnO2 activated peroxymonosulfate process: Effect of water constituents, degradation intermediates and toxicity evaluation. Chemical Engineering Journal, 330, 1390–1400. https://doi.org/10.1016/j.cej.2017.07.137
Divyapriya, G., & Nidheesh, P. V. (2020). Importance of graphene in the electro-Fenton process. ACS Omega, 5(10), 4725–4732. https://doi.org/10.1021/acsomega.9b04201
Dong, P., Chen, X., Guo, M., Wu, Z., Wang, H., Lin, F., et al. (2021). Heterogeneous electro-Fenton catalysis with self-supporting CFP@MnO2-Fe3O4/C cathode for shale gas fracturing flowback wastewater. Journal of Hazardous Materials, 412, 125208. https://doi.org/10.1016/j.jhazmat.2021.125208
Droguett, C., Salazar, R., Brillas, E., Sirés, I., Carlesi, C., Marco, J. F., & Thiam, A. (2020). Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst. Science of the Total Environment, 740, 140154. https://doi.org/10.1016/j.scitotenv.2020.140154
Du, X., Fu, W., Su, P., Cai, J., & Zhou, M. (2020). Internal-micro-electrolysis-enhanced heterogeneous electro-Fenton process catalyzed by Fe/Fe3C@PC core–shell hybrid for sulfamethazine degradation. Chemical Engineering Journal, 398, 125681. https://doi.org/10.1016/j.cej.2020.125681
Eda, G., & Chhowalla, M. (2010). Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Advanced Materials, 22(22), 2392–2415. https://doi.org/10.1002/adma.200903689
El-Ghenymy, A., Garcia-Segura, S., Rodríguez, R. M., Brillas, E., El Begrani, M. S., & Abdelouahid, B. A. (2012). Optimization of the electro-Fenton and solar photoelectro-Fenton treatments of sulfanilic acid solutions using a pre-pilot flow plant by response surface methodology. Journal of Hazardous Materials, 221–222, 288–297. https://doi.org/10.1016/j.jhazmat.2012.04.053
El-Ghenymy, A., Rodríguez, R. M., Arias, C., Centellas, F., Garrido, J. A., Cabot, P. L., & Brillas, E. (2013). Electro-Fenton and photoelectro-Fenton degradation of the antimicrobial sulfamethazine using a boron-doped diamond anode and an air-diffusion cathode. Journal of Electroanalytical Chemistry, 701, 7–13. https://doi.org/10.1016/j.jelechem.2013.04.027
Ganiyu, S. O., Huong Le, T. X., Bechelany, M., Esposito, G., Van Hullebusch, E. D., Oturan, M. A., & Cretin, M. (2017). A hierarchical CoFe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. Journal of Materials Chemistry A, 5(7), 3655–3666. https://doi.org/10.1039/c6ta09100h
Ganiyu, S. O., Zhou, M., & Martínez-Huitle, C. A. (2018). Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Applied Catalysis B: Environmental, 235, 103–129. https://doi.org/10.1016/j.apcatb.2018.04.044
Garcia-Segura, S., & Brillas, E. (2011). Mineralization of the recalcitrant oxalic and oxamic acids by electrochemical advanced oxidation processes using a boron-doped diamond anode. Water Research, 45(9), 2975–2984. https://doi.org/10.1016/j.watres.2011.03.017
Garcia Rodriguez, Y., & CarrascalDomínguez, S. (2017). La influencia del espacio, la ciudad y la Cultura Maker en educación = The influence of space, the city and maker culture in education. ArDIn. Arte, Diseño e Ingeniería, 0(6), 1. https://doi.org/10.20868/ardin.2017.6.3588
Garrido-Ramírez, E. G., Marco, J. F., Escalona, N., & Ureta-Zañartu, M. S. (2016). Preparation and characterization of bimetallic Fe-Cu allophane nanoclays and their activity in the phenol oxidation by heterogeneous electro-Fenton reaction. Microporous and Mesoporous Materials, 225, 303–311. https://doi.org/10.1016/j.micromeso.2016.01.013
Ghanbari, F., & Moradi, M. (2015). A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability improvement. Journal of Environmental Chemical Engineering, 3(1), 499–506. https://doi.org/10.1016/j.jece.2014.12.018
Ghanbarlou, H., Nasernejad, B., NikbakhtFini, M., Simonsen, M. E., & Muff, J. (2020). Synthesis of an iron-graphene based particle electrode for pesticide removal in three-dimensional heterogeneous electro-Fenton water treatment system. Chemical Engineering Journal, 395, 125025. https://doi.org/10.1016/j.cej.2020.125025
Ghasemi, M., Khataee, A., Gholami, P., & CheshmehSoltani, R. D. (2019). Template-free microspheres decorated with Cu-Fe-NLDH for catalytic removal of gentamicin in heterogeneous electro-Fenton process. Journal of Environmental Management, 248, 109236. https://doi.org/10.1016/j.jenvman.2019.07.007
Gong, H., Chu, W., Xu, K., Xia, X., Gong, H., Tan, Y., & Pu, S. (2020). Efficient degradation, mineralization and toxicity reduction of sulfamethoxazole under photo-activation of peroxymonosulfate by ferrate (VI). Chemical Engineering Journal, 389, 124084. https://doi.org/10.1016/j.cej.2020.124084
Gümüş, D., & Akbal, F. (2016). Comparison of Fenton and electro-Fenton processes for oxidation of phenol. Process Safety and Environmental Protection, 103, 252–258. https://doi.org/10.1016/j.psep.2016.07.008
Guo, D., Liu, Y., Ji, H., Wang, C. C., Chen, B., Shen, C., et al. (2021). Silicate-enhanced heterogeneous flow-through electro-fenton system using iron oxides under nanoconfinement. Environmental Science and Technology, 55(6), 4045–4053. https://doi.org/10.1021/acs.est.1c00349
Hammouda, S. B., Fourcade, F., Assadi, A., Soutrel, I., Adhoum, N., Amrane, A., & Monser, L. (2016). Effective heterogeneous electro-Fenton process for the degradation of a malodorous compound, indole, using iron loaded alginate beads as a reusable catalyst. Applied Catalysis B: Environmental, 182, 47–58. https://doi.org/10.1016/j.apcatb.2015.09.007
Hou, L., Zhang, Q., Jérôme, F., Duprez, D., Zhang, H., & Royer, S. (2014). Shape-controlled nanostructured magnetite-type materials as highly efficient Fenton catalysts. Applied Catalysis B: Environmental, 144, 739–749. https://doi.org/10.1016/j.apcatb.2013.07.072
Hu, Y., Lu, Y., Liu, G., Luo, H., Zhang, R., & Cai, X. (2018). Effect of the structure of stacked electro-Fenton reactor on treating nanofiltration concentrate of landfill leachate. Chemosphere, 202, 191–197. https://doi.org/10.1016/j.chemosphere.2018.03.103
Huang, Y., Han, C., Liu, Y., Nadagouda, M. N., Machala, L., O’Shea, K. E., et al. (2018). Degradation of atrazine by ZnxCu1−xFe2O4 nanomaterial-catalyzed sulfite under UV–vis light irradiation: Green strategy to generate SO4[rad]−. Applied Catalysis B: Environmental, 221, 380–392. https://doi.org/10.1016/j.apcatb.2017.09.001
Huong Le, T. X., Bechelany, M., & Cretin, M. (2017). Carbon felt based-electrodes for energy and environmental applications: A review. Carbon, 122, 564–591. https://doi.org/10.1016/j.carbon.2017.06.078
Iglesias, O., Meijide, J., Bocos, E., Sanromán, M. Á., & Pazos, M. (2015). New approaches on heterogeneous electro-Fenton treatment of winery wastewater. Electrochimica Acta, 169, 134–141. https://doi.org/10.1016/j.electacta.2015.04.062
Ismail, S. A., Ang, W. L., & Mohammad, A. W. (2021). Electro-Fenton technology for wastewater treatment: A bibliometric analysis of current research trends, future perspectives and energy consumption analysis. Journal of Water Process Engineering, 40, 101952. https://doi.org/10.1016/j.jwpe.2021.101952
Karimi, S., Shokri, A., & Aghel, B. (2020). Remediation of spent caustic in the wastewater of oil refinery by photo-Fenton process. Archives of Hygiene Sciences, 9(3), 179–188. https://doi.org/10.29252/archhygsci.9.3.179
Khan, K., Benfenati, E., & Roy, K. (2019). Consensus QSAR modeling of toxicity of pharmaceuticals to different aquatic organisms: Ranking and prioritization of the DrugBank database compounds. Ecotoxicology and Environmental Safety, 168, 287–297. https://doi.org/10.1016/j.ecoenv.2018.10.060
Khataee, A., & Hasanzadeh, A. (2018). Modified cathodes with carbon-based nanomaterials for electro-Fenton process. Handbook of Environmental Chemistry, 61, 111–143. https://doi.org/10.1007/698_2017_74
Kim, S., Lee, D. W., & Lee, K. Y. (2014). Direct synthesis of hydrogen peroxide from hydrogen and oxygen over single-crystal cubic palladium on silica catalysts. Journal of Molecular Catalysis A: Chemical, 383–384, 64–69. https://doi.org/10.1016/j.molcata.2013.11.021
Kobya, M., Gebologlu, U., Ulu, F., Oncel, S., & Demirbas, E. (2011). Removal of arsenic from drinking water by the electrocoagulation using Fe and Al electrodes. Electrochimica Acta, 56(14), 5060–5070. https://doi.org/10.1016/j.electacta.2011.03.086
Kumar, A., Rana, A., Sharma, G., Naushad, M., Dhiman, P., Kumari, A., & Stadler, F. J. (2019). Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. Journal of Molecular Liquids, 290, 111177. https://doi.org/10.1016/j.molliq.2019.111177
Lei, H., Li, H., Li, Z., Li, Z., Chen, K., Zhang, X., & Wang, H. (2010). Electro-Fenton degradation of cationic red X-GRL using an activated carbon fiber cathode. Process Safety and Environmental Protection, 88(6), 431–438. https://doi.org/10.1016/j.psep.2010.06.005
Li, J., Wan, Y., Li, Y., Yao, G., & Lai, B. (2019a). Surface Fe(III)/Fe(II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Applied Catalysis B: Environmental, 256, 117782. https://doi.org/10.1016/j.apcatb.2019.117782
Li, X., Liu, F., Zhang, W., Lu, H., & Zhang, J. (2019b). Electrocatalytical oxidation of arsenite by reduced graphene oxide via in-situ electrocatalytic generation of H2O2. Environmental Pollution, 254, 112958. https://doi.org/10.1016/j.envpol.2019.112958
Liang, L., Yu, F., An, Y., Liu, M., & Zhou, M. (2017). Preparation of transition metal composite graphite felt cathode for efficient heterogeneous electro-Fenton process. Environmental Science and Pollution Research, 24(2), 1122–1132. https://doi.org/10.1007/s11356-016-7389-3
Liu, K., Yu, J. C. C., Dong, H., Wu, J. C. S., & Hoffmann, M. R. (2018). Degradation and mineralization of carbamazepine using an electro-Fenton reaction catalyzed by magnetite nanoparticles fixed on an electrocatalytic carbon fiber textile cathode. Environmental Science and Technology, 52(21), 12667–12674. https://doi.org/10.1021/acs.est.8b03916
Luo, T., Feng, H., Tang, L., Lu, Y., Tang, W., Chen, S., et al. (2020). Efficient degradation of tetracycline by heterogeneous electro-Fenton process using Cu-doped Fe@Fe2O3: Mechanism and degradation pathway. Chemical Engineering Journal, 382, 122970. https://doi.org/10.1016/j.cej.2019.122970
Malakootian, M., & Moridi, A. (2017). Efficiency of electro-Fenton process in removing Acid Red 18 dye from aqueous solutions. Process Safety and Environmental Protection, 111, 138–147. https://doi.org/10.1016/j.psep.2017.06.008
Marlina, E., & Purwanto. (2019). Electro-Fenton for industrial wastewater treatment: A review. E3S Web of Conferences, 125, 03003. https://doi.org/10.1051/e3sconf/201912503003
Meijide, J., Dunlop, P. S. M., Pazos, M., & Sanromán, M. A. (2021). Heterogeneous electro-fenton as “Green” technology for pharmaceutical removal: A review. Catalysts, 11(1), 1–22. https://doi.org/10.3390/catal11010085
Meijide, J., Rodríguez, S., Sanromán, M. A., & Pazos, M. (2018). Comprehensive solution for acetamiprid degradation: Combined electro-Fenton and adsorption process. Journal of Electroanalytical Chemistry, 808, 446–454. https://doi.org/10.1016/j.jelechem.2017.05.012
Mi, X., Han, J., Sun, Y., Li, Y., Hu, W., & Zhan, S. (2019a). Enhanced catalytic degradation by using RGO-Ce/WO3 nanosheets modified CF as electro-Fenton cathode: Influence factors, reaction mechanism and pathways. Journal of Hazardous Materials, 367, 365–374. https://doi.org/10.1016/j.jhazmat.2018.12.074
Mi, X., Li, Y., Ning, X., Jia, J., Wang, H., Xia, Y., et al. (2019b). Electro-Fenton degradation of ciprofloxacin with highly ordered mesoporous MnCo2O4-CF cathode: Enhanced redox capacity and accelerated electron transfer. Chemical Engineering Journal, 358, 299–309. https://doi.org/10.1016/j.cej.2018.10.047
Miao, F., Liu, Y., Gao, M., Yu, X., Xiao, P., Wang, M., et al. (2020). Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. Journal of Hazardous Materials, 399, 123023. https://doi.org/10.1016/j.jhazmat.2020.123023
Mohadesi, M., & Shokri, A. (2019). Treatment of oil refinery wastewater by photo-Fenton process using Box-Behnken design method: Kinetic study and energy consumption. International Journal of Environmental Science and Technology, 16(11), 7349–7356. https://doi.org/10.1007/s13762-018-2153-5
Mohadesi, M., & Shokri, A. (2017). Evaluation of fenton and photo-fenton processes for the removal of p-chloronitrobenzene in aqueous environment using Box-Behnken design method. Desalination and Water Treatment, 81, 199–208. https://doi.org/10.5004/dwt.2017.21182
Mohammadi, H., Bina, B., & Ebrahimi, A. (2018). A novel three-dimensional electro-Fenton system and its application for degradation of anti-inflammatory pharmaceuticals: Modeling and degradation pathways. Process Safety and Environmental Protection, 117, 200–213. https://doi.org/10.1016/j.psep.2018.05.001
Monteil, H., Péchaud, Y., Oturan, N., & Oturan, M. A. (2019). A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes: Application to the treatment of pharmaceutical pollutants in water. Chemical Engineering Journal, 376, 119577. https://doi.org/10.1016/j.cej.2018.07.179
Mousset, E., Wang, Z., Hammaker, J., & Lefebvre, O. (2017). Electrocatalytic phenol degradation by a novel nanostructured carbon fiber brush cathode coated with graphene ink. Electrochimica Acta, 258, 607–617. https://doi.org/10.1016/j.electacta.2017.11.104
Munoz, M., de Pedro, Z. M., Casas, J. A., & Rodriguez, J. J. (2015). Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation - A review. Applied Catalysis B: Environmental, 176–177, 249–265. https://doi.org/10.1016/j.apcatb.2015.04.003
Nag, A., Mitra, A., & Mukhopadhyay, S. C. (2018). Graphene and its sensor-based applications: A review. Sensors and Actuators, A: Physical, 270, 177–194. https://doi.org/10.1016/j.sna.2017.12.028
Nair, K. M., Kumaravel, V., & Pillai, S. C. (2021). Carbonaceous cathode materials for electro-Fenton technology: Mechanism, kinetics, recent advances, opportunities and challenges. Chemosphere, 269, 129325. https://doi.org/10.1016/j.chemosphere.2020.129325
Navalon, S., Alvaro, M., & Garcia, H. (2010). Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Applied Catalysis B: Environmental, 99(1–2), 1–26. https://doi.org/10.1016/j.apcatb.2010.07.006
Nidheesh, P. V., Divyapriya, G., Oturan, N., Trellu, C., & Oturan, M. A. (2019). Environmental applications of boron-doped diamond electrodes: 1. Applications in water and wastewater treatment. ChemElectroChem, 6(8), 2124–2142. https://doi.org/10.1002/celc.201801876
Nidheesh, P. V., & Gandhimathi, R. (2012). Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination, 299, 1–15. https://doi.org/10.1016/j.desal.2012.05.011
Nordin, N., Ho, L. N., Ong, S. A., Ibrahim, A. H., Lee, S. L., & Ong, Y. P. (2019). Elucidating the effects of different photoanode materials on electricity generation and dye degradation in a sustainable hybrid system of photocatalytic fuel cell and peroxi-coagulation process. Chemosphere, 214, 614–622. https://doi.org/10.1016/j.chemosphere.2018.09.144
Oriol, R., Sirés, I., Brillas, E., & De Andrade, A. R. (2019). A hybrid photoelectrocatalytic/photoelectro-Fenton treatment of Indigo Carmine in acidic aqueous solution using TiO2 nanotube arrays as photoanode. Journal of Electroanalytical Chemistry, 847, 113088. https://doi.org/10.1016/j.jelechem.2019.04.048
Orkun, M. O., & Kuleyin, A. (2012). Treatment performance evaluation of chemical oxygen demand from landfill leachate by electro-coagulation and electro-fenton technique. Environmental Progress and Sustainable Energy, 31(1), 59–67. https://doi.org/10.1002/ep.10522
Ortiz de la Plata, G. B., Alfano, O. M., & Cassano, A. E. (2010). Decomposition of 2-chlorophenol employing goethite as Fenton catalyst. I. Proposal of a feasible, combined reaction scheme of heterogeneous and homogeneous reactions. Applied Catalysis B: Environmental, 95(1–2), 1–13. https://doi.org/10.1016/j.apcatb.2009.12.005
Oturan, N., Ganiyu, S. O., Raffy, S., & Oturan, M. A. (2017). Sub-stoichiometric titanium oxide as a new anode material for electro-Fenton process: Application to electrocatalytic destruction of antibiotic amoxicillin. Applied Catalysis B: Environmental, 217, 214–223. https://doi.org/10.1016/j.apcatb.2017.05.062
Özcan, A., AtılırÖzcan, A., Demirci, Y., & Şener, E. (2017). Preparation of Fe2O3 modified kaolin and application in heterogeneous electro-catalytic oxidation of enoxacin. Applied Catalysis B: Environmental, 200, 361–371. https://doi.org/10.1016/j.apcatb.2016.07.018
Panizza, M., & Cerisola, G. (2009). Direct and mediated anodic oxidation of organic pollutants. Chemical Reviews, 109(12), 6541–6569. https://doi.org/10.1021/cr9001319
Pham, A. L. T., Doyle, F. M., & Sedlak, D. L. (2012). Inhibitory effect of dissolved silica on H 2O 2 decomposition by iron(III) and manganese(IV) oxides: Implications for H 2O 2-based in situ chemical oxidation. Environmental Science and Technology, 46(2), 1055–1062. https://doi.org/10.1021/es203612d
Pham, A. L. T., Lee, C., Doyle, F. M., & Sedlak, D. L. (2009). A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environmental Science and Technology, 43(23), 8930–8935. https://doi.org/10.1021/es902296k
Pimentel, M., Oturan, N., Dezotti, M., & Oturan, M. A. (2008). Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Applied Catalysis B: Environmental, 83(1–2), 140–149. https://doi.org/10.1016/j.apcatb.2008.02.011
Popat, A., Nidheesh, P. V., Anantha Singh, T. S., & Suresh Kumar, M. (2019). Mixed industrial wastewater treatment by combined electrochemical advanced oxidation and biological processes. Chemosphere, 237, 124419. https://doi.org/10.1016/j.chemosphere.2019.124419
Poza-Nogueiras, V., Rosales, E., Pazos, M., & Sanromán, M. Á. (2018). Current advances and trends in electro-Fenton process using heterogeneous catalysts – A review. Chemosphere, 201, 399–416. https://doi.org/10.1016/j.chemosphere.2018.03.002
Puga, A., Rosales, E., Pazos, M., & Sanromán, M. A. (2020). Prompt removal of antibiotic by adsorption/electro-Fenton degradation using an iron-doped perlite as heterogeneous catalyst. Process Safety and Environmental Protection, 144, 100–110. https://doi.org/10.1016/j.psep.2020.07.021
Ren, G., Zhou, M., Su, P., Yang, W., Lu, X., & Zhang, Y. (2019). Simultaneous sulfadiazines degradation and disinfection from municipal secondary effluent by a flow-through electro-Fenton process with graphene-modified cathode. Journal of Hazardous Materials, 368, 830–839. https://doi.org/10.1016/j.jhazmat.2019.01.109
Ridruejo, C., Alcaide, F., Álvarez, G., Brillas, E., & Sirés, I. (2018). On-site H2O2 electrogeneration at a CoS2-based air-diffusion cathode for the electrochemical degradation of organic pollutants. Journal of Electroanalytical Chemistry, 808, 364–371. https://doi.org/10.1016/j.jelechem.2017.09.010
Segura, Y., Martínez, F., Melero, J. A., & Fierro, J. L. G. (2015). Zero valent iron (ZVI) mediated Fenton degradation of industrial wastewater: Treatment performance and characterization of final composites. Chemical Engineering Journal, 269, 298–305. https://doi.org/10.1016/j.cej.2015.01.102
Shen, J., Li, Y., Zhu, Y., Hu, Y., & Li, C. (2016). Aerosol synthesis of Graphene-Fe 3 O 4 hollow hybrid microspheres for heterogeneous Fenton and electro-Fenton reaction-Fe 3 O 4 Heterogeneous Fenton reaction Electro-Fenton process Degradation of organic pollutants Electrocatalyst. Biochemical Pharmacology, 4, 2469–2476.
Shokri, A. (2017a). Removal of Acid Red 33 from Aqueous Solution by Fenton and Photo Fenton Processes. Journal of Chemical Health Risks, 7, 119–131.
Shokri, A. (2017b). A kinetic study and application of electro-Fenton process for the remediation of aqueous environment containing toluene in a batch reactor. Russian Journal of Applied Chemistry, 90(3), 452–457. https://doi.org/10.1134/S1070427217030193
Shokri, A. (2017c). Investigation of UV/H2O2 process for removal of ortho-toluidine from industrial wastewater by response surface methodology based on the central composite design. Desalination and Water Treatment, 58, 258–266. https://doi.org/10.5004/dwt.2017.0292
Shokri, A. (2019). Employing Sono-Fenton Process for Degradation of 2-Nitrophenol in Aqueous Environment Using Box-Behnken Design Method and Kinetic Study. Russian Journal of Physical Chemistry A, 93(2), 243–249. https://doi.org/10.1134/S003602441902002X
Shokri, A., Bayat, A., Mahanpoor, K., Treat, K. M.-D. W., & 2019, undefined. (2019). Employing fenton-like process for the remediation of petrochemical wastewater through Box–Behnken design method. Desalination and Water Treatment, 166, 135–143. https://doi.org/10.5004/dwt.2019.24634
Shokri, A., & Fard, M. S. (2022a). A critical review in electrocoagulation technology applied for oil removal in industrial wastewater. Chemosphere, 288, 132355. https://doi.org/10.1016/j.chemosphere.2021.132355
Shokri, A., & Fard, M. S. (2022b). A critical review in Fenton-like approach for the removal of pollutants in the aqueous environment. Environmental Challenges, 7, 100534. https://doi.org/10.1016/j.envc.2022b.100534
Shokri, A., Hosseini, J., & Sanavifard, M. (2020). Treatment of synthetic wastewater containing diethyl phthalate through photo-Fenton method by Box-Behnken design. Archives of Hygiene Sciences, 9(2), 121–131. https://doi.org/10.29252/archhygsci.9.2.121
Sklari, S. D., Plakas, K. V., Petsi, P. N., Zaspalis, V. T., & Karabelas, A. J. (2015). Toward the development of a novel electro-Fenton system for eliminating toxic organic substances from water. Part 2. Preparation, characterization, and evaluation of iron-impregnated carbon felts as cathodic electrodes. Industrial and Engineering Chemistry Research, 54(7), 2059–2073. https://doi.org/10.1021/ie5048779
Soltani, F., Navidjouy, N., Khorsandi, H., Rahimnejad, M., & Alizadeh, S. (2021). A novel bio-electro-Fenton system with dual application for the catalytic degradation of tetracycline antibiotic in wastewater and bioelectricity generation. RSC Advances, 11(44), 27160–27173. https://doi.org/10.1039/d1ra04584a
Song, X., Zhang, H., Bian, Z., & Wang, H. (2021). In situ electrogeneration and activation of H2O2 by atomic Fe catalysts for the efficient removal of chloramphenicol. Journal of Hazardous Materials, 412, 125162. https://doi.org/10.1016/j.jhazmat.2021.125162
Sopaj, F., Oturan, N., Pinson, J., Podvorica, F., & Oturan, M. A. (2016). Effect of the anode materials on the efficiency of the electro-Fenton process for the mineralization of the antibiotic sulfamethazine. Applied Catalysis b: Environmental, 199, 331–341. https://doi.org/10.1016/j.apcatb.2016.06.035
Suhan, M. B. K., Shuchi, S. B., Anis, A., Haque, Z., & Islam, M. S. (2020). Comparative degradation study of remazol black B dye using electro-coagulation and electro-Fenton process: Kinetics and cost analysis. Environmental Nanotechnology, Monitoring and Management, 14, 100335. https://doi.org/10.1016/j.enmm.2020.100335
Tang, Q., Wang, D., Yao, D., Yang, C., & Sun, Y. (2016). Heterogeneous electro-Fenton oxidation of p-nitrophenol with a reusable fluffy clump steel wire. Desalination and Water Treatment, 57(33), 15475–15485. https://doi.org/10.1080/19443994.2015.1070758
Thor, S. H., Ho, L. N., Ong, S. A., Abidin, C. Z. A., Heah, C. Y., Nordin, N., et al. (2022). Discovering the roles of electrode distance and configuration in dye degradation and electricity generation in photocatalytic fuel cell integrated electro-Fenton process. Separation and Purification Technology, 278, 119652. https://doi.org/10.1016/j.seppur.2021.119652
Tian, Y., Zhou, M., Pan, Y., Du, X., & Wang, Q. (2021). MoS2 as highly efficient co-catalyst enhancing the performance of Fe0 based electro-Fenton process in degradation of sulfamethazine: Approach and mechanism. Chemical Engineering Journal, 403, 126361. https://doi.org/10.1016/j.cej.2020.126361
Turkay, O., Ersoy, Z. G., & Barışçı, S. (2017). Review—The application of an electro-peroxone process in water and wastewater treatment. Journal of the Electrochemical Society, 164(6), E94–E102. https://doi.org/10.1149/2.0321706jes
Varank, G., YaziciGuvenc, S., & Demir, A. (2018). A comparative study of electrocoagulation and electro-Fenton for food industry wastewater treatment: Multiple response optimization and cost analysis. Separation Science and Technology (Philadelphia), 53(17), 2727–2740. https://doi.org/10.1080/01496395.2018.1470643
Verma, S. K., Khandegar, V., & Saroha, A. K. (2013). Removal of chromium from electroplating industry effluent using electrocoagulation. Journal of Hazardous, Toxic, and Radioactive Waste, 17(2), 146–152. https://doi.org/10.1061/(asce)hz.2153-5515.0000170
Vilar, V. J. P., Moreira, F. C., Ferreira, A. C. C., Sousa, M. A., Gonçalves, C., Alpendurada, M. F., & Boaventura, R. A. R. (2012). Biodegradability enhancement of a pesticide-containing bio-treated wastewater using a solar photo-Fenton treatment step followed by a biological oxidation process. Water Research, 46(15), 4599–4613. https://doi.org/10.1016/j.watres.2012.06.038
Waldt, C. T., Ananthaneni, S., & Rankin, R. B. (2020). Towards quaternary alloy Au–Pd catalysts for direct synthesis of hydrogen peroxide. Materials Today Energy, 16, 100399. https://doi.org/10.1016/j.mtener.2020.100399
Wang, C. T., Chou, W. L., Chung, M. H., & Kuo, Y. M. (2010). COD removal from real dyeing wastewater by electro-Fenton technology using an activated carbon fiber cathode. Desalination, 253(1–3), 129–134. https://doi.org/10.1016/j.desal.2009.11.020
Wang, Y., Zhao, G., Chai, S., Zhao, H., & Wang, Y. (2013). Three-dimensional homogeneous ferrite-carbon aerogel: One pot fabrication and enhanced electro-Fenton reactivity. ACS Applied Materials and Interfaces, 5(3), 842–852. https://doi.org/10.1021/am302437a
Wang, Z., Liu, M., Xiao, F., Postole, G., Zhao, H., & Zhao, G. (2022). Recent advances and trends of heterogeneous electro-Fenton process for wastewater treatment-review. Chinese Chemical Letters, 33(2), 653–662. https://doi.org/10.1016/j.cclet.2021.07.044
Xia, Y., Shang, H., Zhang, Q., Zhou, Y., & Hu, X. (2019). Electrogeneration of hydrogen peroxide using phosphorus-doped carbon nanotubes gas diffusion electrodes and its application in electro-Fenton. Journal of Electroanalytical Chemistry, 840, 400–408. https://doi.org/10.1016/j.jelechem.2019.04.009
Yang, H., Zhou, M., Yang, W., Ren, G., & Ma, L. (2018a). Rolling-made gas diffusion electrode with carbon nanotube for electro-Fenton degradation of acetylsalicylic acid. Chemosphere, 206, 439–446. https://doi.org/10.1016/j.chemosphere.2018.05.027
Yang, W., Zhou, M., Cai, J., Liang, L., Ren, G., & Jiang, L. (2017). Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene. Journal of Materials Chemistry A, 5(17), 8070–8080. https://doi.org/10.1039/c7ta01534h
Yang, W., Zhou, M., & Liang, L. (2018b). Highly efficient in-situ metal-free electrochemical advanced oxidation process using graphite felt modified with N-doped graphene. Chemical Engineering Journal, 338, 700–708. https://doi.org/10.1016/j.cej.2018.01.013
Yang, W., Zhou, M., Oturan, N., Bechelany, M., Cretin, M., & Oturan, M. A. (2020). Highly efficient and stable FeIIFeIII LDH carbon felt cathode for removal of pharmaceutical ofloxacin at neutral pH. Journal of Hazardous Materials, 393, 122513. https://doi.org/10.1016/j.jhazmat.2020.122513
Yang, Y., Qiao, S., Zhou, J., & Quan, X. (2019). A novel porous-carbon-based hollow fiber membrane with electrochemical reduction mediated by in-situ hydroxyl radical generation for fouling control and water treatment. Applied Catalysis B: Environmental, 255, 117772. https://doi.org/10.1016/j.apcatb.2019.117772
Ye, Z., Guelfi, D. R. V., Álvarez, G., Alcaide, F., Brillas, E., & Sirés, I. (2019). Enhanced electrocatalytic production of H2O2 at Co-based air-diffusion cathodes for the photoelectro-Fenton treatment of bronopol. Applied Catalysis B: Environmental, 247, 191–199. https://doi.org/10.1016/j.apcatb.2019.01.029
Ye, Z., Padilla, J. A., Xuriguera, E., Beltran, J. L., Alcaide, F., Brillas, E., & Sirés, I. (2020a). A highly stable metal-organic framework-engineered FeS2/C nanocatalyst for heterogeneous electro-fenton treatment: Validation in wastewater at mild pH. Environmental Science and Technology, 54(7), 4664–4674. https://doi.org/10.1021/acs.est.9b07604
Ye, Z., Padilla, J. A., Xuriguera, E., Brillas, E., & Sirés, I. (2020). Magnetic MIL(Fe)-type MOF-derived N-doped nano-ZVI@C rods as heterogeneous catalyst for the electro-Fenton degradation of gemfibrozil in a complex aqueous matrix. Applied Catalysis B: Environmental, 266, 118604. https://doi.org/10.1016/j.apcatb.2020.118604
Yu, T., & Breslin, C. B. (2020). Graphene-modified composites and electrodes and their potential applications in the electro-fenton process. Materials, 13(10), 2254. https://doi.org/10.3390/ma13102254
Zakeri, H. R., Yousefi, M., Mohammadi, A. A., Baziar, M., Mojiri, S. A., Salehnia, S., & Hosseinzadeh, A. (2021). Chemical coagulation-electro fenton as a superior combination process for treatment of dairy wastewater: Performance and modelling. International Journal of Environmental Science and Technology, 18(12), 3929–3942. https://doi.org/10.1007/s13762-021-03149-w
Zazou, H., Afanga, H., Akhouairi, S., Ouchtak, H., Addi, A. A., Akbour, R. A., et al. (2019). Treatment of textile industry wastewater by electrocoagulation coupled with electrochemical advanced oxidation process. Journal of Water Process Engineering, 28, 214–221. https://doi.org/10.1016/j.jwpe.2019.02.006
Zeng, Z., Khan, A., Wang, Z., Zhao, M., Mo, W., & Chen, Z. (2020). Elimination of atrazine through radical/non-radical combined processes by manganese nano-catalysts/PMS and implications to the structure-performance relationship. Chemical Engineering Journal, 397, 125425. https://doi.org/10.1016/j.cej.2020.125425
Zhang, C., Zhou, M., Ren, G., Yu, X., Ma, L., Yang, J., & Yu, F. (2015). Heterogeneous electro-Fenton using modified iron-carbon as catalyst for 2,4-dichlorophenol degradation: Influence factors, mechanism and degradation pathway. Water Research, 70, 414–424. https://doi.org/10.1016/j.watres.2014.12.022
Zhang, Y., Chen, Z., Wu, P., Duan, Y., Zhou, L., Lai, Y., et al. (2020). Three-dimensional heterogeneous Electro-Fenton system with a novel catalytic particle electrode for Bisphenol A removal. Journal of Hazardous Materials, 393, 120448. https://doi.org/10.1016/j.jhazmat.2019.03.067
Zhao, H., Chen, Y., Peng, Q., Wang, Q., & Zhao, G. (2017). Catalytic activity of MOF(2Fe/Co)/carbon aerogel for improving H2O2 and [rad] OH generation in solar photo–electro–Fenton process. Applied Catalysis B: Environmental, 203, 127–137. https://doi.org/10.1016/j.apcatb.2016.09.074
Zhao, X., Liu, S., & Huang, Y. (2016). Removing organic contaminants by an electro-Fenton system constructed with graphene cathode. Toxicological and Environmental Chemistry, 98(3–4), 530–539. https://doi.org/10.1080/02772248.2015.1123495
Zhong, Y., Liang, X., Tan, W., Zhong, Y., He, H., Zhu, J., et al. (2013). A comparative study about the effects of isomorphous substitution of transition metals (Ti, Cr, Mn, Co and Ni) on the UV/Fenton catalytic activity of magnetite. Journal of Molecular Catalysis A: Chemical, 372, 29–34. https://doi.org/10.1016/j.molcata.2013.01.038
Zhou, M., Zhou, L., Liang, L., Yu, F., & Yang, W. (2018). Cathode modification to improve electro-Fenton performance. Handbook of Environmental Chemistry, 61, 175–203. https://doi.org/10.1007/698_2017_58
Zhou, W., Rajic, L., Chen, L., Kou, K., Ding, Y., Meng, X., et al. (2019). Activated carbon as effective cathode material in iron-free electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption. Electrochimica Acta, 296, 317–326. https://doi.org/10.1016/j.electacta.2018.11.052
Zhou, Z., Cui, Y., Huang, Y., Cao, J., Wu, R., Liang, L., & Huang, Z. (2022). The highly efficient cocatalyst of molybdenum powder enhancing low-cost and stable electro-Fenton system for organic contaminants degradation at wide pH range. Journal of Power Sources, 520, 230860. https://doi.org/10.1016/j.jpowsour.2021.230860
Acknowledgements
The authors are grateful to thank the National Elites Foundation of Iran for financial supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shokri, A., Nasernejad, B. & Sanavi Fard, M. Challenges and Future Roadmaps in Heterogeneous Electro-Fenton Process for Wastewater Treatment. Water Air Soil Pollut 234, 153 (2023). https://doi.org/10.1007/s11270-023-06139-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06139-5