Abstract
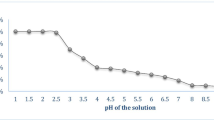
The study is a first-time investigation into the use of Eucalyptus leaves as a low-cost herbal adsorbent for the removal of arsenic (As) and mercury (Hg) from aqueous solutions. The adsorption capacity and efficiency were studied under various operating conditions within the framework of response surface methodology (RSM) by implementing a four-factor, five-level Box–Wilson central composite design (CCD). A pH range of 3–9, contact time (t) of 5–90 min, initial heavy metal (As or Hg) concentration (C 0) of 0.5–3.875 mg/L, and adsorbent dose (m) of 0.5–2.5 g/L were studied for the optimization and modeling of the process. The adsorption mechanism and the relevant characteristic parameters were investigated by four two-parameter (Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich) isotherm models and four kinetic models (Lagergren’s pseudo-first order (PFO), Ho and McKay’s pseudo-second order (PSO), Weber–Morris intraparticle diffusion, and modified Freundlich). The new nonlinear regression-based empirical equations, which were derived within the scope of the study, showed that it might be possible to obtain a removal efficiency for As and Hg above 94% at the optimum conditions of the present process-related variables (pH = 6.0, t = 47.5 min, C 0 = 2.75 mg/L, and m = 1.5 mg/L). Based on the Langmuir isotherm model, the maximum adsorption or uptake capacity of As and Hg was determined as 84.03 and 129.87 mg/g, respectively. The results of the kinetic modeling indicated that the adsorption kinetics of As and Hg were very well described by Lagergren’s PFO kinetic model (R 2 = 0.978) and the modified Freundlich kinetic model (R 2 = 0.984), respectively. The findings of this study clearly concluded that the Persian Eucalyptus leaves demonstrated a higher performance compared to several other reported adsorbents used for the removal of heavy metals from the aqueous environment.






Similar content being viewed by others
References
Akhtar, M., Iqbal, S., Kausar, A., Bhanger, M. I., & Shaheen, M. A. (2010). An economically viable method for the removal of selected divalent metal ions from aqueous solutions using activated rice husk. Colloids and Surfaces B: Biointerfaces, 75(1), 149–155.
Akinbiyi, A. (2000). Removal of lead from aqueous solutions by adsorption using peat moss, M.Sc. thesis, Applied Science in Environmental Systems Engineering, University of Regina, Regina, Saskatchewan, Canada.
Al Rmalli, S. W., Dahmani, A. A., Abuein, M. M., & Gleza, A. A. (2008). Biosorption of mercury from aqueous solutions by powdered leaves of castor tree (Ricinus communis L.) Journal of Hazardous Materials, 152(3), 955–959.
Al-Meshragi, M., Ibrahim, H. G., & Aboabboud, M. M. (2008). Equilibrium and kinetics of chromium adsorption on cement kiln dust. In: Proceedings of the World Congress on Engineering and Computer Science, WCECS 2008, October 22–24, 2008, San Francisco, USA, 54–62.
Al-Subu, M. M. (2002). The interaction effects of cypress (Cupressus sempervirens), cinchona (Eucalyptus longifolia) and pine (Pinushalepensis) leaves on their efficiencies for lead removal from aqueous solutions. Advances in Environmental Research, 6(4), 569–576.
Arivoli, S., Hema, M., Karuppaiah, M., & Saravanan, S. (2008). Adsorption of chromium ion by acid activated low cost carbon-kinetic, mechanistic, thermodynamic and equilibrium studies. Journal of Chemistry, 5(4), 820–831.
Asasian, N., Kaghazchi, T., & Soleimani, M. (2012). Elimination of mercury by adsorption onto activated carbon prepared from the biomass material. Journal of Industrial and Engineering Chemistry, 18(1), 283–289.
Babić, B. M., Milonjić, S. K., Polovina, M. J., Čupić, S., & Kaludjerović, B. V. (2002). Adsorption of zinc, cadmium and mercury ions from aqueous solutions on an activated carbon cloth. Carbon, 40(7), 1109–1115.
Baikousi, M., Georgiou, Y., Daikopoulos, C., Bourlinos, A. B., Filip, J., Zbořil, R., Deligiannakis, Y., & Karakassides, M. A. (2015). Synthesis and characterization of robust zero valent iron/mesoporous carbon composites and their applications in arsenic removal. Carbon, 93, 636–647.
Bajpai, S. K., & Jain, A. (2010). Removal of copper (II) from aqueous solution using spent tea leaves (STL) as a potential sorbent. Water SA, 36(3), 221–228.
Bhowmick, S., Chakraborty, S., Mondal, P., Van Renterghem, W., Van den Berghe, S., Roman-Ross, G., Chatterjee, D., & Iglesias, M. (2014). Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: kinetics and mechanism. Chemical Engineering Journal, 243, 14–23.
Binupriya, A. R., Sathishkumar, M., Swaminathan, K., Kuz, C. S., & Yun, S. E. (2008). Comparative studies on removal of Congo red by native and modified mycelial pellets of Trametes versicolor in various reactor modes. Bioresource Technology, 99(5), 1080–1088.
Bulut, Y., & Aydın, H. (2006). A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination, 194(1–3), 259–267.
Chen, Y. N., Chai, L. Y., & Shu, Y. D. (2008). Study of arsenic (V) adsorption on bone char from aqueous solution. Journal of Hazardous Materials, 160(1), 168–172.
Dehghani, M. H., Mohammadi, M., Mohammadi, M. A., Mahvi, A. H., Yetilmezsoy, K., Bhatnagar, A., Bhatnagar, A., Heibati, B., & McKay, G. (2016). Equilibrium and kinetic studies of trihalomethanes adsorption onto multi-walled carbon nanotubes. Water, Air, & Soil Pollution, 227(9), 1–17.
Demirbas, E., Kobya, M., Senturk, E., & Ozkan, T. (2004). Adsorption kinetics for the removal of chromium (VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water SA, 30(4), 533–539.
Dhawane, S. H., Kumar, T., & Halder, G. (2015). Central composite design approach towards optimization of flamboyant pods derived steam activated carbon for its use as heterogeneous catalyst in transesterification of Hevea brasiliensis oil. Energy Conversion and Management, 100, 277–287.
Dutta, S., Bhattacharyya, A., De, P., Ray, P., & Basu, S. (2009). Removal of mercury from its aqueous solution using charcoal-immobilized papain (CIP). Journal of Hazardous Materials, 172(2), 888–896.
ECETOC (European Centre for Ecotoxicology and Toxicology of Chemicals) (2017). Technical Report 123, Freundlich isotherms. http://www.ecetoc.org/. Accessed 17.02.10.
Erhayem, M., Al-Tohami, F., Mohamed, R., & Ahmida, K. (2015). Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. American Journal of Analytical Chemistry, 6, 1–10.
Fakhri, A. (2015). Investigation of mercury (II) adsorption from aqueous solution onto copper oxide nanoparticles: optimization using response surface methodology. Process Safety and Environmental Protection, 93, 1–8.
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management, 92(3), 407–418.
Genç-Fuhrman, H., Tjell, J. C., & McConchie, D. (2004). Increasing the arsenate adsorption capacity of neutralized red mud (Bauxsol). Journal of Colloid and Interface Science, 271(2), 313–320.
Giri, A. K., & Patel, R. K. (2011). Studies on the removal of Hg(II) from water by activated adsorbent prepared from Eichhornia crassipes biomass, In: Proceedings of the 3rd International CEMEPE & SECOTOX Conference, Skiathos Island, Greece.
Goel, J., Kadirvelu, K., & Rajagopal, C. (2004). Competitive sorption of Cu (II), Pb (II) and Hg (II) ions from aqueous solution using coconut shell-based activated carbon. Adsorption Science & Technology, 22(3), 257–273.
Gupta, S. S., & Bhattacharyya, K. G. (2011). Kinetics of adsorption of metal ions on inorganic materials: a review. Advances in Colloid and Interface Science, 162(1), 39–58.
Gupta, A., Vidyarthi, S. R., & Sankararamakrishnan, N. (2015). Concurrent removal of As(III) and As(V) using green low cost functionalized biosorbent—Saccharum officinarum bagasse. Journal of Environmental Chemical Engineering, 3(1), 113–121.
Hadavifar, M., Bahramifar, N., Younesi, H., Rastakhiz, M., Li, Q., Yu, J., & Eftekhari, E. (2016). Removal of mercury (II) and cadmium (II) ions from synthetic wastewater by a newly synthesized amino and thiolated multi-walled carbon nanotubes. Journal of the Taiwan Institute of Chemical Engineers, 67, 397–405.
Hassan, S. S., Awwad, N. S., & Aboterika, A. H. (2008). Removal of mercury (II) from wastewater using camel bone charcoal. Journal of Hazardous Materials, 154(1), 992–997.
Heibati, B., Rodriguez-Couto, S., Turan, N. G., Ozgonenel, O., Albadarin, A. B., Asif, M., Tyagi, I., Agarwal, S., & Gupta, V. K. (2015). Removal of noxious dye—Acid Orange 7 from aqueous solution using natural pumice and Fe-coated pumice stone. Journal of Industrial and Engineering Chemistry, 31, 124–131.
Henke, K. (2009). Arsenic: environmental chemistry, health threats and waste treatment. Hoboken: John Wiley & Sons.
Hewings, G. J. D., Changnon, S., & Dridi, C. (2002). Testing for the significance of extreme weather and climate event on the state economies, the Regional Economics Application Laboratory, REAL 00–T– 6, S (pp. 1–16). Urbana: Mathews.
Ho, Y. S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70(2), 115–124.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465.
Ho, Y. S., & McKay, G. (2002). Application of kinetic models to the sorption of copper (II) on to peat. Adsorption Science & Technology, 20(8), 797–815.
Iakovleva, E., Maydannik, P., Ivanova, T. V., Sillanpää, M., Tang, W. Z., Mäkilä, E., Salonen, J., Gubal, A., Ganeev, A. A., Kamwilaisak, K., & Wang, S. (2016). Modified and unmodified low-cost iron-containing solid wastes as adsorbents for efficient removal of As (III) and As (V) from mine water. Journal of Cleaner Production, 133, 1095–1104.
Inbaraj, B. S., & Sulochana, N. (2006). Mercury adsorption on a carbon sorbent derived from fruit shell of Terminalia catappa. Journal of Hazardous Materials, 133(1), 283–290.
Kadirvelu, K., & Namasivayam, C. (2003). Activated carbon from coconut coirpith as metal adsorbent: adsorption of Cd (II) from aqueous solution. Advances in Environmental Research, 7(2), 471–478.
Kanel, S. R., Manning, B., Charlet, L., & Choi, H. (2005). Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Environmental Science & Technology, 39(5), 1291–1298.
Kang, M., Kawasaki, M., Tamada, S., Kamei, T., & Magara, Y. (2000). Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination, 131(1–3), 293–298.
Kavitha, D., & Namasivayam, C. (2007). Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresource Technology, 98(1), 14–21.
Krishnan, K. A., Sreejalekshmi, K. G., & Baiju, R. S. (2011). Nickel (II) adsorption onto biomass based activated carbon obtained from sugarcane bagasse pith. Bioresource Technology, 102(22), 10239–10247.
Kurniawan, T. A., Chan, G. Y., Lo, W. H., & Babel, S. (2006). Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Science of the Total Environment, 366(2), 409–426.
Lin, T. F., & Wu, J. K. (2001). Adsorption of arsenite and arsenate within activated alumina grains: equilibrium and kinetics. Water Research, 35(8), 2049–2057.
Liu, H. L., Lan, Y. W., & Cheng, Y. C. (2004). Optimal production of sulphuric acid by Thiobacillus thiooxidans using response surface methodology. Process Biochemistry, 39(12), 1953–1961.
Liu, M., Hou, L. A., Xi, B., Zhao, Y., & Xia, X. (2013). Synthesis, characterization, and mercury adsorption properties of hybrid mesoporous aluminosilicate sieve prepared with fly ash. Applied Surface Science, 273, 706–716.
Liu, J., Huang, X., Liu, J., Wang, W., Zhang, W., & Dong, F. (2014). Adsorption of arsenic(V) on bone char: batch, column and modeling studies. Environmental Earth Sciences, 72(6), 2081–2090.
Mangwandi, C., Suhaimi, S. N., Liu, J. T., Dhenge, R. M., & Albadarin, A. B. (2016). Design, production and characterisation of granular adsorbent material for arsenic removal from contaminated wastewater. Chemical Engineering Research and Design, 110, 70–81.
Mohan, D., & Pittman, C. U. (2007). Arsenic removal from water/wastewater using adsorbents—a critical review. Journal of Hazardous Materials, 142(1), 1–53.
Mondal, D. K., Nandi, B. K., & Purkait, M. K. (2013). Removal of mercury (II) from aqueous solution using bamboo leaf powder: equilibrium, thermodynamic and kinetic studies. Journal of Environmental Chemical Engineering, 1(4), 891–898.
Mosaferi, M., Taghipour, H., Hassani, A. M., Borghei, M., Kamali, Z., & Ghadirzadeh, A. (2008). Study of arsenic presence in drinking water sources: a case study. Iranian Journal of Health and Environment, 1(1), 19–28.
Mosaferi, M., Nemati, S., Khataee, A., Nasseri, S., & Hashemi, A. A. (2014). Removal of arsenic (III, V) from aqueous solution by nanoscale zero-valent iron stabilized with starch and carboxymethyl cellulose. Journal of Environmental Health Science and Engineering, 12(1), 74.
Mousavi, S. Z., & Lotfi, Z. (2011). Removal of nickel and cadmium from aqueous solution by modified magnetic nanoparticles. Journal of Water and Wastewater, 1(95), 2–11.
Mudasir, M., Karelius, K., Aprilita, N. H., & Wahyuni, E. T. (2016). Adsorption of mercury (II) on dithizone-immobilized natural zeolite. Journal of Environmental Chemical Engineering, 4(2), 1839–1849.
Nethaji, S., Sivasamy, A., & Mandal, A. B. (2013). Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. International Journal of Environmental Science and Technology, 10(2), 231–242.
Ngah, W. W., & Hanafiah, M. A. K. M. (2008). Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: kinetic, equilibrium and thermodynamic studies. Biochemical Engineering Journal, 39(3), 521–530.
Nguyen, C., & Do, D. D. (2001). The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon, 39(9), 1327–1336.
Noori Sepehr, M. N., Yetilmezsoy, K., Marofi, S., Zarrabi, M., Ghaffari, H. R., Fingas, M., & Foroughi, M. (2014). Synthesis of nanosheet layered double hydroxides at lower pH: optimization of hardness and sulfate removal from drinking water samples. Journal of the Taiwan Institute of Chemical Engineers, 45(5), 2786–2800.
Organic Information Services Pvt Ltd. (2017). 6 Impressive eucalyptus benefits. Available from: https://www.organicfacts.net/health-benefits/herbs-and-spices/eucalyptus.html. Accessed September 20, 2017.
Patel, H., & Vashi, R. T. (2014). COD and BOD removal from textile wastewater using naturally prepared adsorbents and their activation forms using sulphuric acid. In H. A. Aziz & A. Mojiri (Eds.), Wastewater engineering: advanced wastewater treatment systems. Penang: IJSR Publications.
Pehlivan, E., Tran, H. T., Ouédraogo, W. K. I., Schmidt, C., Zachmann, D., & Bahadir, M. (2013). Sugarcane bagasse treated with hydrous ferric oxide as a potential adsorbent for the removal of As(V) from aqueous solutions. Food Chemistry, 138(1), 133–138.
Qi, J., Zhang, G., & Li, H. (2015). Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresource Technology, 193, 243–249.
Rajamohan, N., Rajasimman, M., Rajeshkannan, R., & Saravanan, V. (2014). Equilibrium, kinetic and thermodynamic studies on the removal of aluminum by modified Eucalyptus camaldulensis barks. Alexandria Engineering Journal, 53(2), 409–415.
Reed, B. E., Vaughan, R., & Jiang, L. (2000). As (III), As (V), Hg, and Pb removal by Fe-oxide impregnated activated carbon. Journal of Environmental Engineering, 126(9), 869–873.
Rocha, C. G., Zaia, D. A. M., da Silva Alfaya, R. V., & da Silva Alfaya, A. A. (2009). Use of rice straw as biosorbent for removal of Cu (II), Zn (II), Cd (II) and Hg (II) ions in industrial effluents. Journal of Hazardous Materials, 166(1), 383–388.
Samadi, M. T., Salimi, M., & Saghi, M. H. (2010). Comparison of granular activated carbon, natural clinoptilolite zeolite, and anthracite packed columns in removing mercury from drinking water. Water and Wastewater, 20(4), 54–59.
Sampranpiboon, P., & Feng, X. (2016). Kinetic models on chromium (VI) adsorption onto carbonized oil palm kernel with potassium hydroxide activation. International Journal of Advances in Chemical Engineering and Biological Sciences, 3(1), 66–71.
Sathishkumar, M., Binupriya, A. R., Vijayaraghavan, K., & Yun, S. I. (2007). Two and three-parameter isothermal modeling for liquid-phase sorption of Procion Blue H-B by inactive mycelial biomass of Panus fulvus. Journal of Chemical Technology and Biotechnology, 82(4), 389–398.
Shabbiri, K., Adnan, A., Jamil, S., Ahmad, W., Noor, B., & Rafique, H. M. (2012a). Medium optimization of protease production by Brevibacterium linens DSM 20158, using statistical approach. Brazilian Journal of Microbiology, 43(3), 1051–1061.
Shabbiri, K., Adnan, A., Noor, B., & Jamil, S. (2012b). Optimized production, purification and characterization of alpha amylase by Brevibacterium linens DSM 20158, using bio-statistical approach. Annals of Microbiology, 62(2), 523–532.
Silva, H. S., Ruiz, S. V., Granados, D. L., & Santángelo, J. M. (2010). Adsorption of mercury (II) from liquid solutions using modified activated carbons. Materials Research, 13(2), 129–134.
Sinha, A., & Khare, S. K. (2012). Mercury bioremediation by mercury accumulating Enterobacter sp. cells and its alginate immobilized application. Biodegradation, 23(1), 25–34.
Smedley, P. L., & Kinniburgh, D. G. (2013). Arsenic in groundwater and the environment. In Essentials of medical geology, Springer Netherlands, pp. 279–310.
Thanawatpoontawee, S., Imyim, A., & Praphairaksit, N. (2016). Iron-loaded zein beads as a biocompatible adsorbent for arsenic (V) removal. Journal of Industrial and Engineering Chemistry, 43, 127–132.
Thompson, N. E., Emmanuel, G. C., George, N. I., & Adamu, I. K. (2015). Modelling of the kinetic and equilibrium sorption behaviour of crude oil on HDTMAB modified nigerian nanoclays. International Journal of Scientific & Technology Research, 4(2), 106–114.
Türk, T., Alp, İ., & Deveci, H. (2009). Adsorption of As (V) from water using nanomagnetite. Journal of Environmental Engineering, 136(4), 399–404.
Ulmanu, M., Marañón, E., Fernández, Y., Castrillón, L., Anger, I., & Dumitriu, D. (2003). Removal of copper and cadmium ions from diluted aqueous solutions by low cost and waste material adsorbents. Water, Air, & Soil Pollution, 142(1), 357–373.
Varank, G., Demir, A., Yetilmezsoy, K., Top, S., Sekman, E., & Bilgili, M. S. (2012). Removal of 4-nitrophenol from aqueous solution by natural low-cost adsorbents. Indian Journal of Chemical Technology, 19(1), 7–25.
Vimonses, V., Lei, S., Jin, B., Chow, C. W., & Saint, C. (2009). Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chemical Engineering Journal, 148(2), 354–364.
Wang, Q., Hou, Y., Xu, Z., Miao, J., & Li, G. (2008). Optimization of cold-active protease production by the psychrophilic bacterium Colwellia sp. NJ341 with response surface methodology. Bioresource Technology, 99(6), 1926–1931.
Wang, S. Y., Tang, Y. K., Chen, C., Wu, J. T., Huang, Z., Mo, Y. Y., Zhang, K. X., & Chen, J. B. (2015). Regeneration of magnetic biochar derived from eucalyptus leaf residue for lead (II) removal. Bioresource Technology, 186, 360–364.
Weng, Y. H., Chaung-Hsieh, L. H., Lee, H. H., Li, K. C., & Huang, C. P. (2005). Removal of arsenic and humic substances (HSs) by electro-ultrafiltration (EUF). Journal of Hazardous Materials, 122(1), 171–176.
Yaghmaeian, K., Mashizi, R. K., Nasseri, S., Mahvi, A. H., Alimohammadi, M., & Nazmara, S. (2015). Removal of inorganic mercury from aquatic environments by multi-walled carbon nanotubes. Journal of Environmental Health Science and Engineering, 13(1), 55.
Yaghmaeian, K., Jaafarzadeh, N., Nabizadeh, R., Rasoulzadeh, H., & Akbarpour, B. (2016). Evaluating the performance of modified adsorbent of zero valent iron nanoparticles–chitosan composite for arsenate removal from aqueous solutions. Iranian Journal of Health and Environment, 8(4), 535–548.
Yakout, S. M., & Elsherif, E. (2010). Batch kinetics, isotherm and thermodynamic studies of adsorption of strontium from aqueous solutions onto low cost rice-straw based carbons. Carbon: Science and Technology, 3(1), 144–153.
Yardim, M. F., Budinova, T., Ekinci, E., Petrov, N., Razvigorova, M., & Minkova, V. (2003). Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere, 52(5), 835–841.
Yenial, Ü., Bulut, G., & Ali Sirkeci, A. (2014). Arsenic removal by adsorptive flotation methods. CLEAN–Soil, Air, Water, 42(11), 1567–1572.
Yetilmezsoy, K., & Demirel, S. (2008). Artificial neural network (ANN) approach for modeling of Pb(II) adsorption from aqueous solution by Antep pistachio (Pistacia vera L.) shells. Journal of Hazardous Materials, 153(3), 1288–1300.
Yetilmezsoy, K., Demirel, S., & Vanderbei, R. J. (2009). Response surface modeling of Pb (II) removal from aqueous solution by Pistacia vera L.: Box–Behnken experimental design. Journal of Hazardous Materials, 171(1), 551–562.
Zeng, L. (2004). Arsenic adsorption from aqueous solutions on an Fe (III)-Si binary oxide adsorbent. Water Quality Research Journal of Canada, 39(3), 267–275.
Zhuang, Z., Huang, L., Wang, F., & Chen, Z. (2015). Effects of cyclodextrin on the morphology and reactivity of iron-based nanoparticles using Eucalyptus leaf extract. Industrial Crops and Products, 69, 308–313.
Acknowledgements
The authors would like to thank the personnel of the Environmental Health Laboratory, Tehran University of Medical Sciences.
Funding
The authors would like to thank Tehran University of Medical Sciences for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Alimohammadi, M., Saeedi, Z., Akbarpour, B. et al. Adsorptive Removal of Arsenic and Mercury from Aqueous Solutions by Eucalyptus Leaves. Water Air Soil Pollut 228, 429 (2017). https://doi.org/10.1007/s11270-017-3607-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3607-y