Abstract
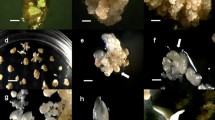
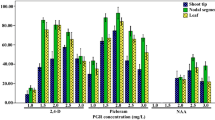
An efficient plant regeneration protocol has been developed from embryogenic callus derived from cotyledonary explants of Clitoria ternatea Linn., an important medicinal climber species. Optimum embryogenic callus (75 %) was induced on Murashige and Skoog (MS) medium supplemented with 2 mg/l 2, 4-dichlorophenoxyacetic acid (2, 4-D). On subculturing the callus on MS medium supplemented with 2 mg/l 6-benzyladenine (BA) and 0.5 mg/l α-naphthalene acetic acid (NAA), 61 % of cultures responded with a mean number of 22 somatic embryos per gram callus at different stages of development after 45 days of culture. The addition of higher concentrations of sucrose and abscisic acid (ABA) significantly increased the embryogenic response. MS medium supplemented with 2 mg/l BA, 0.2 mg/l NAA and 4 % of sucrose resulted in 76 % of cultures responding with a mean number of 28 embryos per one gram callus. The highest embryogenic response, frequency of 83 % and mean number of 37 embryos per gram callus, was observed when the MS medium was supplemented with 2 mg/l BA, 0.2 mg/l NAA, and 3 mg/l ABA. Synthetic seeds were produced by encapsulating embryos in calcium alginate gel. The gel contained MS medium with 3 % of sucrose, 1.0 mg/l BA and 0.2 mg/l NAA. The synthetic seeds germinated on MS medium supplemented with BA (1.0-4.0 mg/l) alone or in combination with NAA (0.1–0.7 mg/l) or indole-3-butyric acid (IBA; 0.1–0.7 mg/l). The highest synthetic seed germination (92 %) was observed on MS medium supplemented with 2 mg/l BA and 0.5 mg/l NAA. The synthetic seeds were stored at 4 °C and lab conditions (25 ± 2 °C) up to 5 months. The synthetic seeds kept at 4 °C showed 86 % viability even after 5 months of storage. Both somatic embryos and synthetic seeds germinated and were transferred to soil successfully.





Similar content being viewed by others
Abbreviations
- 2, 4-D:
-
2, 4-Dichlorophenoxyacetic acid
- ABA:
-
Abscisic acid
- BA:
-
6-benzyladenine
- Kn:
-
Kinetin
- MS:
-
Murashige and Skoog
- NAA:
-
α-naphthalene acetic acid
- IBA:
-
Indole-3-butyric acid
References
Anonymous (1988) The wealth of India: a dictionary of Indian raw materials and industrial products, vol. II. New Delhi: Publication and Information Directorate. CSIR, India, pp 608–643
Anthony JM, Senaratna T, Dixon T, Sivasithamparam K (2004) Somatic embryogenesis for mass propagation of Ericaceae: a case study with Leucopogon verticillatus. Plant Cell Tiss Org Cult 76:137–146
Attree SM, Fowke LC (1993) Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tiss Org Cult 35:1–35
Banerjee SK, Chakravarti RN (1963) Taraxerol from Clitoria ternatea. Bull Calcutta School Trop Med 11:106–107
Banerjee SK, Chakravarti RN (1964) Taraxerone from Clitoria ternatea. Bull Calcutta School Trop Med 12:23
Barik DP, Naik SK, Mudgal A, Chand PK (2007) Rapid plant regeneration through in vitro axillary shoot proliferation of butterfly pea (Clitoria ternatea L.): a twinning legume. In Vitro Cell Dev Biol Plant 43:144–148
Baskaran P, Van Staden J (2012) Somatic embryogenesis of Merwilla plumbea (Lindl.) Speta. Plant Cell Tiss Organ Cult doi:10.1007/s11240-012-0118-9
Bhojwani SS, Razdan MK (1996) Plant tissue culture: theory and practice, a revised edition. Elsevier, Amsterdam, pp 1–766
Cangahuala-Inocente GC, Vesco LLD, Steinmacher D, Torres AC, Guerra MP (2007) Improvements in somatic embryogenesis protocol in Feijoa (Acca sellowiana (Berg) Burret): induction, conversion and synthetic seeds. Sci Hort 111:228–234
Cardosa JC, Martinelli AP, Latado RR (2012) Somatic embryogenesis from ovaries of sweet orange cv. Tobias. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-0073-x
Castillo B, Smith MA, Yadava UL (1988) Plant regeneration from encapsulated somatic embryos of Carica papaya L. Plant Cell Rep 17:172–176
Cruz GS, Canhoto JM, Abreu MAV (1990) Somatic embryogenesis and plant regeneration from zygotic embryos of Feijoa sellowiana Berg. Plant Sci 66:263–270
DeWald SG, Litz RE, Moore GA (1989) Optimizing somatic embryo production in mango. J Am Soc Hortic Sci 114:837–841
Dhanalakshmi S, Lakshmanan KK (1992) In vitro somatic embryogenesis and plant regeneration in Clitoria ternatea. J Exp Bot 43:213–219
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Fei SZ, Riordan T, Read P (2002) Stepwise decrease of 2, 4-D and addition of BA in subculture medium stimulated shoot regeneration and somatic embryogenesis in buffalo grass. Plant Cell Tiss Organ Cult 70:275–279
Ganapathi TR, Srinivas L, Suprasanna P, Bapat VA (2001) Regeneration of plants from alginate-encapsulated somatic embryos of banana cv. Rasthali (Musa spp. AAB group). In Vitro Cell Dev Biol Plant 37:178–181
George EF, Sherrington PD (1984) In vitro Plant propagation by tissue culture. Eversley, Exegetics Ltd, England, pp 39–71
Gerdakaneh M, Mozafari AA, Khalighi A, Sioseh-mardah A (2009) The Effects of carbohydrate source and concentration on somatic embryogenesis of strawberry (Fragaria x ananassa Duch.). Am-Eurasian J Agric Environ Sci 6:76–80
Groll J, Gray VM, Mycock DJ (2002) Development of Cassava (Manihot esculenta Crantz.) somatic embryos during culture with abscisic acid and activated charcoal. J Plant Physiol 159:437–443
Huda AKMN, Bari MA, Rahman M (2009) Asexual propagation of eggplant (Solanum melongena L.) through encapsulated axillary buds. Plant Tissue Cult Biotech 19:263–288
Jain NN, Ohal CC, Shroff SK, Bhutada RH, Somani RS, Kasture VS, Kasture SB (2003) Clitoria ternatea and the CNS. Pharmac Biochem Behav 75:529–536
Jang DS, Cuendet M, Pawlus AD, Kardono LB, Kawanishi K, Farnsworth NR, Fong HH, Pezzuto JM, Kinghorn AD (2004) Potential cancer chemopreventive constituents of the leaves of Macaranga triloba. Phytochem 65:345–350
Karami O, Deljou A, Esna-ashari M, Ostat-ahmadi P (2006) Effect of sucrose concentrations on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Sci Hort 110:340–344
Kitto SL, Janick J (1982) Polyox as an artificial seed coat for a sexual embryos. Hort Sci 17:448
Kong DM, Preece JE, Shen HL (2012) Somatic embryogenesis in immature cotyledons of Manchurian ash (Fraxinus mandshurica Rupr.). Plant Cell Tiss Organ Cult 108:485–492
Kulkarni C, Pattanshetty JR, Amruthraj G (1988) Effect of alcoholic extract of Clitoria ternatea Linn. On central nervous system in rodents. Ind J Exp Biol 26:957–960
Lecouteux C, Lai FM, McKersie BD (1993) Maturation of alfalfa (Medicago sativa L.) somatic embryos by abscisic acid, sucrose and chilling stress. Plant Sci 94:207–213
Lee JH, Lee KT, Yang JH, Baek NI, Kim DK (2004) Acetylcholinesterase inhibitors from the twigs of Vaccinium oldhami Miquel. Arch Pharm Res 27:53–56
Lin LC, Chou CJ, Kuo YC (2001) Cytotoxic principles from Ventilago leiocarpa. J Nat Prod 64:674–676
Linossier L, Veisseire P, Cailloux F, Coudret A (1997) Effects of abscisic acid and high concentrations of PEG on Hevea brasiliensis somatic embryos development. Plant Sci 124:183–191
Litz RE (1988) Somatic embryogenesis from cultured leaf explants of the tropical tree Euphoria longan Stend. J Plant Physiol 132:190–193
Litz RE, Gray DJ (1995) Somatic embryogenesis for agricultural improvement. World J Microbiol Biotech 11:416–425
Malabadi RB, Nataraja K (2001) Shoot regeneration of leaf explant of Clitoria ternatea L. cultured in vitro. Phytomorp 51:169–171
Mathur J, Ahuja PS, Lal N, Mathur AK (1989) Propagation of Valeriana wallichii DC using encapsulated apical and axial shoot buds. Plant Sci 60:111–116
Michael Gomez SM, Kalamani A (2003) Butterfly pea (Clitoria ternatea L.): a nutritive multipurpose forage legume for the tropics: an overview. Pak J Nutr 2:374–379
Mondal TK, Bhattacharya A, Sood A, Ahuja PS (2002) Factors affecting germination and conversion frequency of somatic embryos of Tea (Camellia sinensis (L.) O. Kuntze. J Plant Physiol 159:1317–1321
Murashige T (1977) Plant cell and organ cultures as horticultural practices. Acta Hort 78:17
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naik DG, Mujumdar AM, Waghole RJ, Misar AV, Bligh SW, Bashall A, Crowder J (2004) Taraxer-14-en-3beta-ol, an anti-inflammatory compound from Sterculia foetida L. Planta Med 70:68–69
Nuno-Ayala A, Rodriguez-Garay B, Gutierrez-Mora A (2012) Somatic embryogenesis in Jarilla hetrophylla (Caricaceae). Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-0070-0
Nyende AB, Schittenhelm S, Mix-Wagner G, Greef JM (2003) Production, storability and regeneration of shoot tips of potato (Solanum tuberosum L.) encapsulated in calcium alginate hollow beads. In Vitro Cell Dev Biol Plant 39:540–544
Nyende AB, Schittenhelm S, Mix-Wagner G, Greef JM (2005) Yield and canopy development of field grown potato plants derived from synthetic seeds. Europ J Agron 22:175–184
Panday NK, Tewari KC, Tewari RN, Joshi GC, Pande VN, Pandey G (1993) Medicinal plants of Kumaon Himalaya: strategies for conservation. In: Dhar U (ed) Himalayan biodiversity conservation strategies, no. 3. Nanital, Himavikas Publications, India, pp 293–302
Parimaladevi B, Boominathan R, Mandal SC (2003) Anti-inflammatory, analgesic and antipyretic properties of Clitoria ternatea root. Fitoterapia 74:345–349
Pattnaik S, Chand PK (2000) Morphogenic response of the alginate-encapsulated axillary buds from in vitro shoot cultures of six mulberries. Plant Cell Tiss Organ Cult 60:177–185
Rai KS, Murthy KD, Karanth KS, Rao MS (2001) Clitoria ternatea Linn. root extract treatment during growth spurt period enhances learning and memory in rats. Ind J Physiol Pharmac 45:305–313
Rai KS, Murthy KD, Karanth KS, Nalini K, Rao MS, Srinivasan KK (2002) Clitoria ternatea root extract enhances acetylcholine content in rat hippocampus. Fitoterapia 73:685–689
Ratanasanobon K, Seaton KA (2010) Development of in vitro plant regenertion of Australian native waxflowers (Chamelaucium spp.) via somatic embryogenesis. Plant Cell Tiss Organ Cult 100:59–64
Ricci AP, Filho FAM, Januzzi BM, Piedade SMS (2002) Somatic embryogenesis in Citrus sinensis, C. reticulate and C. nobilis × C. deliciosa. Sci Agricol 59:41–46
Rout GR (2005) Micropropagation of Clitoria ternatea Linn. (Fabaceae)—an important medicinal plant. In Vitro Cell Dev Biol Plant 41:516–519
Sahrawat AK, Chand S (2001) Continuous somatic embryogenesis and plant regeneration from hypocotyl segments of Psoralea corylifolia Linn., an endangered and medicinally important Fabaceae plant. Curr Sci 81:1328–1331
Shahana S, Gupta SC (2002) Somatic embryogenesis in Sesbania sesban var. bicolor: A multipurpose fabaceous woody species. Plant Cell Tiss Organ Cult 69:289–292
Shahzad A, Faisal M, Anis M (2007) Micropropagation through excised root culture of Clitoria ternatea and comparison between in vitro–regenerated plants and seedlings. Ann Appl Biol 150:341–349
Sharma RK, Bhagwan D (1988) Agnivesa’s Caraka Samhita, vol. 3: Chaukhambha Orientalia. Varanasi, India, p 46
Shirin F, Hossain M, Kabir MF, Roy M, Sarker SR (2007) Callus induction and plant regeneration from internodal and leaf explants of four potato (Solanum tuberosum L.) cultivers. World J Agri Sci 3:1–6
Shu Y, Ying-Cai Y, Hong-Hui L (2005) Plant regeneration through somatic embryogenesis from callus cultures of Dioscorea zingiberensis. Plant Cell Tiss Organ Cult 80:157–161
Singh J, Tiwari KN (2012) In vitro plant regeneration from decapitated embryonic axes of Clitoria ternatea L: an important medicinal plant. Ind Crops Prod 35:224–229
Singh S, Tanwer BS, Khan M (2011) Callus induction and in vivo and in vitro comparative study of primary metabolites of Withania Somnifera. Adv App Sci Res 2:47–52
Sivanesan I, Lim MY, Jeong BR (2011) Somatic embryogenesis and plant regeneration from leaf and petiole explants of Campanula punctata Lam. var. rubriflora Makino. Plant Cell Tiss Organ Cult 107:365–369
Sivarajan VV, Balachandran I (1994) Ayurvedic drugs and their plant sources. Vol. 97. Oxford IBH, New Delhi, pp 289–290
Skirvin RM, Norton M, McPheeters KD (1993) Somaclonal variation: has it proved useful for plant improvement. Acta Hort 336:333–340
Song GQ, Loskutov AV, Sink KC (2007) Highly efficient Agrobacterium tumefaciens mediated transformation of celery (Apium graveolens L.) through somatic embryogenesis. Plant Cell Tiss Organ Cult 88:193–200
Takasaki M, Konoshima T, Tokuda H, Masuda K, Arai Y, Shiojima K, Ageta H (1999) Anti-carcinogenic activity of Taraxacum plant. II. Biol Pharm Bull 22:606–610
Taranalli AD, Cheeramkuzhy TC (2003) Influence of Clitoria ternatea extracts on memory and cerebro cholinergic activity in rats. Pharm Biol 38:51–56
Tetteroo FAA, Hoekstra FA, Karssen CM (1995) Induction of complete desiccation tolerance in carrot (Daucus carota) embryoids. J Plant Physiol 145:349–356
Thorpe TA, Harry IS, Kumar PP (1991) Application of micropropagation to forestry. In: Debergh PC, Zimmerman RH (eds) Micropropagation technology and application. Kluwer Academic Publishers, Dordrecht, pp 311–336
Wani M, Pande S, More N (2010) Callus induction studies in Tridax procumbens L. Int J Biotechnol App 2:11–14
Wu HC, Toit ESD, Reinhardt EF (2007) A protocol for direct somatic embryogenesis of Protea cynaroides L. using zygotic embryos and cotyledon tissues. Plant Cell Tiss Organ Cult 89:217–224
Yang JL, Seong ES, Kim MJ, Ghimire BK, Kang WH, Yu CY, Li CH (2010) Direct somatic embryogenesis from pericycle cells of broccoli (Brassica oleracea var. italica) root explants. Plant Cell Tiss Organ Cult 100:49–58
Yoganarasimhan SN (2000) Medicinal plants of India. Vol. 2. Interline Publishing Co, Bangalore, pp 146–147
Zhou S, Brown DCW (2006) High efficiency plant production of North American ginseng via somatic embryogenesis from cotyledon explants. Plant Cell Rep 25:166–173
Acknowledgments
We thank the Principal for providing with necessary laboratory facilities. TDT acknowledges the financial assistance from UGC, Government of India, in the form of a major research project (Project no. 38-233/2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna Kumar, G., Thomas, T.D. High frequency somatic embryogenesis and synthetic seed production in Clitoria ternatea Linn. Plant Cell Tiss Organ Cult 110, 141–151 (2012). https://doi.org/10.1007/s11240-012-0138-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0138-5