Abstract
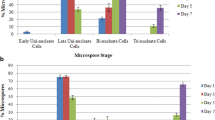
In an attempt to discover the biological basis of microspore derived embryogenesis, the effect of the antimicrotubule agent colchicine on anther and free microspore embryogenesis was investigated. The microtubule inhibitor colchicine promoted embryogenesis from cultured anthers, both with regard to the number of anthers responding and the number of embryos being produced per anther. A similar promotional response was also observed with cultured microspores. Although the parameters for cultured anthers and free microspores differed, administration of the drug for a short period immediately prior to pollen mitosis I seems to exert the maximum promotional effect. Of the five cultivars of Brassica napus studied, all responded to colchicine treatment. However, the drug did release more embryogenic potential in poor-responding varieties (i.e. Lirawell and Optima) than in the highest responding variety (Topas). Colchicine also resulted in increased embryogenic response in microspores cultured at lower temperatures.
These results are considered in terms of models proposed to explain the switch in microspore development from a gametophytic to a sporophytic pathway. The use ofcolchicine as agent to promote embryogenesis in previously recalcitrant species other than Brassica is also discussed.
Similar content being viewed by others
References
Barnabas B & Kovacs G (1990) Comparison of different methods for production of dihaploid plants from Triticum aestivum anther cultures. In: Abstr. VII Inter. Cong. Plant Tiss. Cell Cult. Abstract. No. A7–10
Bennett M & Hughes W (1972) Additional mitosis of wheat pollen induced by Ethrel. Nature 240: 566–568.
Bhojwani S, Dunwell J & Sunderland N (1973) Nucleic-acid and protein contents of embryogenic tobacco pollen. J. Exp. Bot. 24: 863–871
Charzynska M & Pennenko I (1976) Inhibition of cytokinesis in the microspores of Tradescantia bracteata by caffeine. Act. Soc. Bot. Pol. 45: 469–479.
Chuong P & Beversdorf W (1985) High frequency embryogenesis through isolated microspore culture in Brassica napus and Brassica carinata. Plant Sci. 39: 219–226.
Dickinson H & Heslop-Harrison J (1971) The mode of growth of the inner layer of the pollen grain exine in Lilium. Cytobios 4: 233–243
Dickinson H & Sheldon J (1984) A radial system of microtubules extending between the nuclear envelope and the plasma membrane during early male haplophase in flowering plants. Planta 161: 86–90
Dunwell J & Sunderland N (1974) Pollen ultrastructure in anther cultures of Nicotiana tabacum. II. Changes associated with embryogenesis. J. Expt. Bot. 25: 363–373
Dunwell J, Cornish M & DeCourcel A (1985) Influence of genotype, plant growth temperature and anther incubation temperature on microspore embryo production in Brassica napus. J. Exp. Bot. 36: 679–689
Fan Z, Armstrong K & Keller W (1988) Development of microspores in vivo and in vitro in Brassica napus. Protoplasma 147: 191–199
Foroughi-Wehr B & Friedt W (1984) Rapid production of recombinant barley yellow mosaic virus-resistant Hordeum vulgare lines by anther culture. Theor. Appl. Genet. 67: 377–382
Guha S & Maheshwari S (1964) In vitro production of embryos from anthers of Datura. Nature 204: 297
Hause G, Hause B & Van Lammeren A (1992) Microtubules and actin filament configurations during microspore and pollen development in Brassica napus cv. Topas. Can. J. Bot. 70: 1369–1376
Hause B, Hause G, Pechan P & Van Lammeren A (1993) Cytoskeletal changes and induction of embryogenesis in microspore and pollen cultures of Brassica napus. Cell Biol. Inter. 17: 153–168
Hu H & Huang B (1987) Application of pollen-derived plants to crop improvement. Int. Rev. Cyt. 107: 293–311
Katsuta J & Shibaoka H (1988) The roles of the cytoskeleton and the cell wall in nuclear positioning in tobacco BY-2 cells. Plant Cell Physiol. 29: 403–413
Katsuta J, Hashiguchi Y & Shibaoka H (1990) The role of the cytoskeleton in positioning of the nucleus in premitotic tobaco BY-2 cells. J. Cell Sci. 95: 413–422
Keller W & Armstrong K (1978) High frequency production of microspore-derived plants from Brassica napus anther cultures. Z. Pflanzenzüchtg 80: 100–108
Keller W, Rojhathy T & Lacapra J (1975) In vitro, production of plants from pollen in Brassica campestris. Can. J. Genet. Cytol. 17: 655–666
Lichter R (1985) From microspores to rape plants: a tentative way to low glucosinolate strains. In: Sorensen H (Ed) Cruciferous Crops (268–277). Nijhoff/Dr. W. Junk, Publ., Dordrecht
Meijer M & Simmonds D (1988) Microtubule organization during the development of the mitotic apparatus in cultured mesophyll protoplasts of higher plants — an immunofluorescence microscopic study. Physiol. Plant. 74: 225–232
Nitsch C (1977) Culture of isolated microspores. In: Reinert J & Bajaj Y (Eds) Plant Cell, Tissue and Organ Culture (pp 268–278). Springer, Berlin
Ockendon D (1984) Anther culture in Brussels sprouts (Brassica oleracea var. gemmifera) I. Embryo yields and plant regeneration. Ann. Appl. Biol. 105: 285–291
Olmsted J & Borisy G (1973) Microtubules. Ann. Rev. Biochem. 42: 507–540
Pechan P & Schell J (1990) Molecular changes associated with the commitment phase of microspore embryogenesis. In: Nijkamp HJ, Van derPlas LH & van Aartrijk J (Eds) Progress in Plant Cellular and Molecular Biology (pp 407–409). Kluwer Acad. Publ., Dordrecht.
Pechan P, Bartels D, Brown D & Schell J (1991) Messenger-RNA and protein changes associated with induction of Brassica microspore embryogenesis. Planta 184: 161–165
Raghavan V (1977) Patterns of DNA synthesis during pollen embryogenesis in henbane. J. Cell Biol. 73: 521–526
Raghavan V (1979) An autoradiographic study of RNA synthesis during pollen embryogenesis in Hyoscyamus niger (henbane). Amer. J. Bot. 66: 784–795
Raghavan V (1984) Protein synthetic activity during normal pollen development and during induced pollen embryogenesis in Hyoscyamus niger. Can. J. Bot. 62: 2493–2513
Sangwan R (1978) Amino acid metabolism in cultured anthers of Datura metel. Biochem. Physiol. Pflanzen. 173: 355–364
Sangwan-Norreel B (1983) Male gametophyte nuclear DNA content evolution during androgenic induction in Datura innoxia. Z. Pflanzenphysiol. 111: 47–54
Sax K (1937) Effect of variations in temperature on nuclear division in Tradescantia. Amer. J. Bot. 24: 218–225
Sheldon J & Dickinson H (1986) Pollen wall formation in Lilium: the effect of chaotropic agents, and the organization of the microtubular cytoskeleton during pattern development. Planta 168: 11–23
Simmonds D, Gervais C & Keller W (1991) Embryogenesis from microspores of embryogenic and non-embryogenic lines of Brassica napus. In: Mc Gregor DI(Ed) Proceedings GCIRC 8th International Rapeseed Congress, Saskatoon, Sask, Canada (pp 306–311)
Srivastave P & Johri B (1988) Pollen embryogenesis. J. Palynology 23: 83–99
Tanaka I & Ito M (1981) Control of division in explanted microspores of Tulipa gesneriana. Protoplasma 108: 329–340
Terasaka O & Nitsch T (1990) Unequal cell division and chromatin differention in pollen grain cells. II Microtubule dynamics associated with unequal cell division. Bot. Mag. Tokyo 103: 133–140
Tiwari S (1989) Cytoskeleton during pollen development in Tradescantia virginiana: a study employing chemical fixation, freezesubstitution, immunofluorescence, and colchicine administration. Can. J. Bot. 67: 1244–1253
Traas J, Doonan J, Rawlins D, Shaw P, Watts J & Lloyd C (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J. Cell Biol. 105: 387–395
Vergne P, Riccardim F, Beckert M & Dumas C (1990) Detection of androgenesis-related proteins in maize. In: Nijkamp HJ, van derPlas LH & van Aartrijk J (Eds) Progress in Plant Cellular and Molecular Biology (pp 416–421). Kluwer Acad. Publ., Dordrecht
Wernicke W, Ros M & Jung G (1990) Microtubules and the first cell cycle in cultured mesophyll protoplasts of Nicotiana. In: Nijkamp HJ, van derPlas LH & van Aartrijk J (Eds) Progress in Plant Cellular and Molecular Biology (pp 538–543). Kluwer Acad. Publ., Dordrecht
Zaki M & Dickinson H (1990) Structural changes during the first divisions of embryos resulting from anther and free microspore culture in Brassica napus. Protoplasma 156: 149–162
Zaki M & Dickinson H (1991) Microspore-derived embryos in Brassica: the significance of division symmetry in pollen mitosis I to embryogenic development. Sex. Plant Rep. 4: 48–55
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zaki, M., Dickinson, H. Modification of cell development in vitro: The effect of colchicine on anther and isolated microspore culture in Brassica napus . Plant Cell Tiss Organ Cult 40, 255–270 (1995). https://doi.org/10.1007/BF00048132
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00048132