Abstract
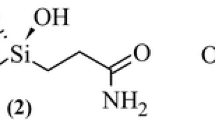
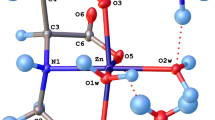
We report a DFT study (M06L/cc-pVDZ) of the interactions between the Si(OH)2 group in three simplified gem-silanediols [i.e., N-[dihydroxy(methyl)silyl] methyl}formamide (DHSF), 3-[dihydroxy (methyl) silyl] propanamide (DHSP), and 3,3′-(dihydroxysilanediyl)dipropanamide (DHSDP)], which have a similar structure to silanediol-based inhibitors of metalloproteases, and simplified active site models: [Zn(Imdz)3–OH2]2+ and [Zn(Imdz)2R–OH2]2+, where R can be a formaldehyde, an acetone, or an acetic acid molecule. These models partly resemble the structure of the first coordination sphere of some metalloproteases (e.g., angiotensin I converting enzyme and thermolysin). Different types of bonding patterns were found for the systems into study. The three related silanediols may coordinate with the zinc dication in monodentate, pseudo-bidentate, and pseudo-tridentate way. Pseudo-bidentate interaction was reported to be that corresponding to the silanediol transition-state-analog of the thermolysin enzyme as confirmed by the X-ray structural study (Juers et al., Biochemistry 44:16524–16528, 2005). The binding ability of the mentioned silanediols was determined as the energy of the water displacement reaction for the mentioned active sites models in gas phase and in water solution (PCM model). The calculated binding energies point out to the higher strength of the pseudo-bidentate Zn2+–MBG interaction. Moreover, DHSDP ligand is calculated to be the strongest MBG for Zn2+ in both active sites models. NBO population analysis and the AIM methodology were implemented as a tool for evaluating electronic structure of the complexes. The results obtained may point out to the fact that the higher the electronic delocalization around the metal center is, the stronger the interaction between the MBG and the active site, bringing about a higher binding energy.






Similar content being viewed by others
References
Leung D, Abbenante G, Fairlie DP (2000) J Med Chem 43:305–341
Natesh R, Schwager SLU, Evans HR, Sturrock ED, Acharya KR (2004) Biochemistry 43:8718–8724
Turner AJ, Hooper NM (2002) Trends Pharmacol Sci 23:177–183
Burnier M, Brunner HR (2000) Lancet 355:637–645
Massie BM (1998) Lancet 352(Suppl I):SI29–SI33
Bruinsm M, Janssen A, Boom R (2001) Appl Biochem Biotechnol 90:155–186
Gupta SP (2007) Chem Rev 107:3042–3087
Morgan BP, Scholtz JM, Ballinger MD, Zipkin ID, Bartlett PA (1991) J Am Chem Soc 113:297–307
Tian GR, Wang S-H, Wang S-F, Meng L-Q, Li H, Zeng Z-H, Jin J-Y (2011) Med Chem Commun 2:698–700
Borkakoti N (2004) Biochem Soc Trans 32:17–20
Ondetti MA, Rubin B, Cushman DW (1977) Science 196:441–444
Patchett AA, Harris E, Tristram EW, Wyvratt MJ, Wu MT, Taub D, Peterson ER, Ikeler TJ, Broeke JT, Payne LG, Ondeyka DL, Thorsett ED, Greenlee WJ, Lohr NS, Hoffsommer RD, Joshua H, Ruyle WV, Rothrock JW, Aster SD, Maycock AL, Robinson FM, Hirschmann R, Sweet CS, Ulm EH, Gross DM, Vassil TC, Stone CA (1980) Nature 288:280–283
Sieburth SM, Nittoli T, Mutahu A, Guo L (1998) Angew Chem Int Ed 37:811–814
Chen C-A, Sieburth SMcN, Glekas A, Hewitt GW, Trainor GL, Erickson-Viitanen S, Garber SS, Cordova B, Jeffry S, Klabe RM (2001) Chem Biol 8:1161–1166
Mutahi MWa, Nittoli T, Guo L, Sieburth SMcN (2002) J Am Chem Soc 124:7363–7375
Kim J, Glekas A, Sieburth SMcN (2002) Bioorg Med Chem Lett 12:3625–3627
West R, Wilson LS, Powell DL (1979) J Organomet Chem 178:5–9
Damrauer R, Kass SR, Depuy CH (1988) Organometallics 7:637–640
Frison G, Ohanessian G (2007) J Comput Chem 29:416–433
Wyvratt MJ, Patchett AA (1985) Med Res Rev 5:483–531
Kimura E (2001) Acc Chem Res 34:171–179
Šramko M, Garaj V, Remko M (2008) J Mol Struct (Theochem) 869:19–28
Dedachi K, Hassan Khan MT, Sylte I, Kurita N (2009) Chem Phys Lett 479:290–295
Dedachi K, Hirakawa T, Fujita S, Hassan Khan MT, Sylte I, Kurita N (2011) J Comput Chem 32:3047–3057
Hirakawa T, Fijita S, Ohyama T, Dedachi K, Hassan Khan MT, Sylte I, Kurita N (2012) J Mol Graph Model 33:1–11
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP (1999) Nature 401:188–193
Somoza JR, Skene RJ, Katz BA, Mol C, Ho JD, Jennings AJ, Luong C, Arvai A, Buggy JJ, Chi E, Tang J, Sang BC, Verner E, Wynands R, Leahy EM, Dougan DR, Snell G, Navre M, Knuth MW, Swanson RV, McRee DE, Tari LW (2004) Structure 12:1325–1334
Cheng F, Zhang R, Luo X, Shen J, Li X, Gu J, Zhu W, Shen J, Sagi I, Ji R, Chen K, Jiang H (2002) J Phys Chem B 106:4552–4559
Smieško M, Remko MJ (2003) Biomol Struct Dyn 20:759–770
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101–194118
Woon DE, Dunning TH Jr (1993) J Chem Phys 98:1358–1371
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Amin EA, Truhlar DG (2008) J Chem Theory Comput 4:75–80
Sorkin A, Iron MA, Truhlar DG (2008) J Chem Theory Comput 4:307–315
Cramer CJ, Truhlar DG (2009) Phys Chem Chem Phys 11:10757–10816
Zeng Y, Wang S, Feng H, Xie Y, King RB (2011) Int J Mol Sci 12:2216–2231
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 Revision B.1. Gaussian Inc., Wallingford
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117–129
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Glendening ED, Reed AE, Carperter JE, Weinhold F (1988) NBO Version 3.1, Madison
Bader RFW (1990) Atoms in molecules. A quantum theory. Oxford University Press, New York
Biegler-König FW, Schönbohm J (2002) J. Comput Chem 23:1489–211494
Biegler-König F, Schönbohm J (2000) AIM2000. University of Applied Sciences, Bielefeld
MacCall KA, Huang C-C, Fierke CA (2000) J Nutr 130:1437S–1446S
Rodríguez Ortega PG, Montejo M, López González JJ (2013) (Results to be published)
Juers DH, Kim J, Matthews BW, Sieburth SM (2005) Biochemistry 44:16524–16528
Ho J, Klamt A, Coote ML (2010) J Phys Chem A 114:13442–13444
Popelier P (2000) Atoms in molecules. An introduction. Pearson Education, London
Acknowledgments
The authors thank Andalusian government for funding (FQM173). P.G.R.O. thanks Spanish Ministerio de Educación for a Ph.D. studentship (AP2009-3949) supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez Ortega, M.P.G., Montejo, M. & López González, J.J. Interaction models of the Si(OH)2 functionality with Zn2+ cation in simplified biological environments: a DFT study. Struct Chem 25, 127–138 (2014). https://doi.org/10.1007/s11224-013-0258-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0258-5