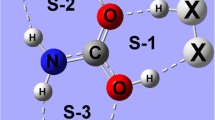
Abstract
The crystal structure of NH4[Zn(cma)(H2O)2]·H2O (cma3– = N-carboxymethylaspartate(3–)) is determined by single crystal X-ray structure analysis. The orthorombic crystals (P212121, a = 7.7901(4) Å, b = 11.2368(4) Å, c = 13.2048(5) Å, α = β = γ = 90°, Z = 4) were obtained from the reaction mixture in the form of racemic conglomerate. The single crystal X-ray structure analysis revealed the maximum deviation of bond angles around the Zn atom from an ideal octahedral geometry 14.09° with ∑ = 67.23° and Θ = 236.69°. Intermolecular interactions are based mainly on a moderate N–H⋯O and O–H⋯O hydrogen bonds. The structure shares similar structural features with other structures containing aspartates and their derivatives as a ligands. The results of using different HAR methods based on semi-empirical (B3LYP) and non-empirical (PBE0) global hybrid GGA DFT functionals were compared.
Graphical Abstract
The crystal structure of NH4[Zn(cma)(H2O)2]·H2O (cma3– = N-carboxymethylaspartate(3–)) is determined by single crystal X-ray structure analysis. The orthorombic crystals (P212121, a = 7.7901(4) Å, b = 11.2368(4) Å, c = 13.2048(5) Å, α = β = γ = 90°, Z = 4) were obtained from the reaction mixture in the form of racemic conglomerate.




Similar content being viewed by others
Data Availability
The deposition numbers CCDC 2152269–2152272 contain the supplementary crystallographic data for this paper (including structure factors). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif or Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033. Crystallographic data can be also obtained from Crystallography Open Database (COD) via https://www.crystallography.net/384cod/search.html under the COD ID 3000339–3000342.
References
Maderová J, Pavelčík F, Marek J (2002) N-(Carboxymethyl)aspartic acid. Acta Crystallogr Sect E Struct Rep Online 58:o469–o470. https://doi.org/10.1107/s1600536802004117
Loginova ES, Nikolskii VM, Tolkacheva LN, Lukyanova NI (2016) Synthesis and some properties of complexones, succinic acid derivatives. Russ Chem Bull 65:2206–2210. https://doi.org/10.1007/s11172-016-1569-7
Kaparullina EN, Doronina NV, Ezhov VA, Trotsenko YA (2012) EDTA degradation by cells of Chelativorans oligotrophicus immobilized on a biofilter. Appl Biochem Microbiol 48:396–400. https://doi.org/10.1134/S0003683812040096
Smirnova TI, Khizhnyak SD, Nikol’skii VM et al (2017) Degradation of complexons derived from succinic acid under UV radiation. Russ J Appl Chem 90:507–511. https://doi.org/10.1134/S1070427217040024
Cherrier MV, Cavazza C, Bochot C et al (2008) Structural characterization of a putative endogenous metal chelator in the periplasmic nickel transporter NikA. Biochemistry 47:9937–9943. https://doi.org/10.1021/bi801051y
Knyazeva NE (2002) Complexation of Zn2+ with N-(carboxymethyl)aspartic acid. Russ J Inorg Chem 47:718–720
Knyazeva NE, Nikol’skii VM, Gorelov IP, (2002) A pH- and redox-potentiometric study of equilibria between Fe(II), Fe(III), and N-(carboxymethyl)aspartic acid. Russ J Coord Chem 28:127–130. https://doi.org/10.1023/A:1014236202268
Knyazeva NE, Nikol’skii VM, Alekseev VG et al (2002) Complexation of Fe2+ with N-(carboxymethyl)aspartic and iminodisuccinic acids. Russ J Inorg Chem 47:216–219
Nikol’skiǐ VM, Knyazeva NE, Gorelov IP (2004) Mn2+ and Cu2+ complexation with N-(carboxymethyl) aspartic acid by potentiometry. Russ J Inorg Chem 49:799–801
Gorelov IP, Knyazeva NE, Nikol’skiǐ VM (2004) Complexation of Co2+ and Ni2+ with N-(carboxymethyl)aspartic acid by potentiometry. Russ J Inorg Chem 49:802–804
Maderová J, Marek J, Pavelčík F (2003) [N-(Carboxylatomethyl)aspartato(3-)]-(ethylenediamine)cobalt(III) trihydrate. Acta Crystallogr Sect C Cryst Struct Commun 59:1996–1998. https://doi.org/10.1107/S0108270103006449
Capelli SC, Bürgi H-B, Dittrich B et al (2014) Hirshfeld atom refinement. IUCrJ 1:361–379. https://doi.org/10.1107/S2052252514014845
Rigaku Oxford Diffraction (2018) CrysAlisPro Software system, version 1.171.39.46. Rigaku Corporation, Oxford
Pavelcik F (1999) XFPA 98: a program for automatic structure determination and automatic refinement. J Appl Crystallogr 32:839–840. https://doi.org/10.1107/S0021889899007451
Pavelčík F, Pivovarčíková O (2002) Patterson oriented automatic structure determination: superposition pseudosymmetry. J Appl Crystallogr 35:526–532
Božeková I (2020) Selection of testing structures and preliminary testing of XFPB software. Comenius University in Bratislava, Faculty of Natural Sciences, Bratislava
Dolomanov OV, Bourhis LJ, Gildea RJ et al (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Kleemiss F, Dolomanov OV, Bodensteiner M et al (2021) Accurate crystal structures and chemical properties from NoSpherA2. Chem Sci 12:1675–1692. https://doi.org/10.1039/d0sc05526c
Neese F (2018) Software update: the ORCA program system, version 4.0. Wiley Interdiscip Rev Comput Mol Sci 8:4–9. https://doi.org/10.1002/wcms.1327
Bernardinelli G, Flack H (1985) Least-squares absolute-structure refinement. Practical experience and ancillary calculations. Acta Crystallogr Sect A 41:500–511
Hooft RWW, Straver LH, Spek AL (2008) Determination of absolute structure using Bayesian statistics on Bijvoet differences. J Appl Crystallogr 41:96–103. https://doi.org/10.1107/S0021889807059870
Marchivie M, Guionneau P, Létard J-F, Chasseau D (2005) Photoinduced spintransition: the role of the iron(II) environment distortion. Acta Crystallogr Sect B Struct Sci 61:25–28. https://doi.org/10.1107/S0108768104029751
McCusker JK, Rheingold AL, Hendrickson DN (1996) Variable-temperature studies of laser-initiated 5T2 → 1A1 intersystem crossing in spin-crossover complexes: empirical correlations between activation parameters and ligand structure in a series of polypyridyl ferrous complexes. Inorg Chem 35:2100–2112. https://doi.org/10.1021/ic9507880
Ketkaew R, Tantirungrotechai Y, Harding P et al (2021) OctaDist: a tool for calculating distortion parameters in spin crossover and coordination complexes. J Chem Soc Dalt Trans 50:1086–1096. https://doi.org/10.1039/D0DT03988H
Colomb G, Bernauer K (1977) Stereoselectivity in reactions of metal complexes. V). Synthesis of mixed-ligand cobalt(III) complexes with (S)-aspartic-N-monoacetic acid and different amino-acids. Helv Chim Acta 60:459–467
Spackman PR, Turner MJ, McKinnon JJ et al (2021) CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J Appl Crystallogr 54:1006–1011. https://doi.org/10.1107/S1600576721002910
Jayatilaka D, Grimwood DJ (2003) Tonto: A Fortran based object-oriented system for quantum chemistry and crystallography. Lect Notes Comput Sci (Including Subser Lect Notes Artif Intell Lect Notes Bioinformatics) 2660:142–151. https://doi.org/10.1007/3-540-44864-0_15
Plevová K, Kisszékelyi P, Vargová D et al (2021) Diastereoselective double C−H functionalization of chiral ferrocenes with heteroaromatics. Chem A Eur J 27:15501–15507. https://doi.org/10.1002/chem.202102624
Peňaška T, Modrocká V, Stankovianska K et al (2022) Organocatalytic diastereodivergent enantioselective formal oxa-diels-alder reaction of unsaturated ketones with enoates under liquid-assisted grinding conditions. Chemsuschem 15:e202200028. https://doi.org/10.1002/cssc.202200028
Allen FH, Bruno IJ (2010) Bond lengths in organic and metal-organic compounds revisited: X–H bond lengths from neutron diffraction data. Acta Crystallogr Sect B Struct Sci 66:380–386. https://doi.org/10.1107/S0108768110012048
Cooper RI, Thompson AL, Watkin DJ (2010) CRYSTALS enhancements: dealing with hydrogen atoms in refinement. J Appl Crystallogr 43:1100–1107. https://doi.org/10.1107/S0021889810025598
Hammershøi A, Sargeson AM, Steffen WL (1984) Reactivity studies of chelated maleate ion: stereoselectivity and structural correlations. J Am Chem Soc 106:2819–2837. https://doi.org/10.1021/ja00322a016
Oonishi I, Sato S, Saito Y (1975) The crystal structure of calcium cis(N)–trans(O6)-bis-(L-aspartato)cobaltate(III)–water (2/15). Acta Crystallogr Sect B 31:1318–1324. https://doi.org/10.1107/S0567740875005134
Sekizaki M (1978) The crystal structure of L-asparaginato-D-aspartatocobalt(III) monohydrate. Bull Chem Soc Jpn 51:1991–1995. https://doi.org/10.1246/bcsj.51.1991
Flaig R, Koritsanszky T, Zobel D, Luger P (1998) Topological analysis of the experimental electron densities of amino acids. 1. D, L-Aspartic acid at 20 K. J Am Chem Soc 120:2227–2238. https://doi.org/10.1021/ja972620e
Madsen D, Pattison P (2000) N-Methyl-DL-aspartic acid monohydrate. Acta Crystallogr Sect C Cryst Struct Commun 56:1157–1158. https://doi.org/10.1107/S0108270100008593
Acknowledgements
This work was supported by the Scientific Grant Agency of the Ministry of Education of Slovak Republic and of Slovak Academy of Sciences VEGA 2/0019/19 and by the Operation Program of Integrated Infrastructure for the project, UpScale of Comenius University Capacities and Competence in Research, Development and Innovation, ITMS2014+: 313021BUZ3, co-financed by the European Regional Development Fund. The authors thank to prof. František Pavelčík for supplying development version of his XFPB software and Mgr. Ivana Božeková for her vast amount of work during preliminary testing phase.
Author information
Authors and Affiliations
Contributions
YPR refined and described the synthesis of the ligand. JC and YPR prepared the title compound. ER with YPR did the X-ray structure determination and prepared the figures. All authors contributed to the main manuscript text and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chrappová, J., Pateda, Y.R. & Rakovský, E. Synthesis and Crystal Structure Analysis of NH4[Zn(cma)(H2O)2]·H2O Using IAM and HAR Approaches. J Chem Crystallogr 53, 228–235 (2023). https://doi.org/10.1007/s10870-022-00961-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-022-00961-1