Abstract
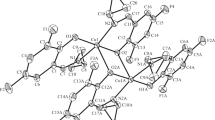
Two new Schiff base copper(II) complexes, [Cu2L2] (I) and [Cu2L(NCNCN)2]n (II), where L is the dianionic form of N,N'-bis(5-fluorosalicylidene)-1,3-propanediamine (H2L), were synthesized and characterized by elemental analyses, infrared and electronic spectroscopy, and single crystal X-ray determinations (CIF files CCDC. nos. 2000415 (I) and 2000416 (II)). Complex I is a phenolate bridged dinuclear copper(II) compound. Complex II is a phenolate and dicyanoamide bridged polynuclear copper(II) compound. In both complexes, the Cu atoms are in square pyramidal coordination. The Schiff base ligand coordinates to the Cu atoms through the phenolate O and imino N atoms. The Schiff base H2L and the two complexes showed high selectivity and antimicrobial activities against a number of the bacteria and fungi.


Similar content being viewed by others
REFERENCES
Buta, I., Cseh, L., Cretu, C., et al., Inorg. Chim. Acta, 2018, vol. 475, p. 133.
Vieru, V., Pasatoiu, T.D., Ungur, L., et al., Inorg. Chem., 2016, vol. 55, no. 23, p. 12158.
Balashova, T.V., Baranov, E.V., Fukin, G.K., et al., Russ. J. Coord. Chem., 2019, vol. 45, no. 10, p. 712. https://doi.org/10.1134/S107032841909001X
Esfahani, M.H. and Behzad, M., J. Coord. Chem., 2020, vol. 73, no. 1, p. 154.
Burlov, A.S., Vlasenko, V.G., Koshchienko, Y.V., et al., Polyhedron, 2018, vol. 154, p. 123.
Burlov, A.S., Koshchienko, Y.V., Kiskin, M.A., et al., J. Mol. Struct., 2016, vol. 1104, p. 7.
Venkateswarlu, K., Ganji, N., Daravath, S., et al., Polyhedron, 2019, vol. 171, p. 86.
Burlov, A.S., Shepelenko, E.N., Vasilchenko, I.S., et al., Russ. J. Coord. Chem., 2006, vol. 32, no. 12, p. 879. https://doi.org/10.1134/S1070328406120049
Burlov, A.S., Vlasenko, V.G., Koshchienko, Y.V., et al., Appl. Organomet. Chem., 2020, vol. 34, no. 1, е5302. https://doi.org/10.1002/aoc.5302
Zhu, Y.Y., Wang, C.F., Yan, K., et al., J. Coord. Chem., 2016, vol. 69, no. 16, p. 2493.
Qiu, X.Y., Gu, Y.T., Li, Y.T., et al., Russ. J. Coord. Chem., 2017, vol. 43, no. 5, p. 331. https://doi.org/10.1134/S1070328417050062
Zhang, M., Xian, D.-M., Li, H.-H., et al., Aust. J. Chem., 2012, vol. 65, no. 4, p. 343.
Gopalakrishnan, M., Thanusu, J., Kanagarajan, V., et al., J. Enzym. Inhib. Med. Chem., 2009, vol. 24, no. 1, p. 52.
SMART (version 5.628) and SAINT (version 6.02), Madison: Bruker AXS Inc., 1998.
Sheldrick, G.M., SADABS, Program for Empirical Absorption Correction of Area Detector, Göttingen: Univ. of Göttingen, 1996.
Sheldrick, G.M., SHELXTL V5.1, Software Reference Manual, Madison: Bruker AXS, Inc., 1997.
Meletiadis, J., Meis, J.F., Mouton, J.W., et al., J. Clin. Microbiol., 2000, vol. 38, no. 8, p. 2949.
Pradhan, A., Haldar, S., Mallik, K.B., et al., Inorg. Chim. Acta, 2019, vol. 484, p. 197.
Peng, D.L., Russ. J. Coord. Chem., 2019, vol. 45, no. 10, p. 734. https://doi.org/10.1134/S107032841910004X
Jana, K., Maity, T., Mahapatra, T.S., et al., Transit. Met. Chem., 2017, vol. 42, no. 1, p. 69.
Talukder, P., Shit, S., Sasmal, A., et al., Polyhedron, 2011, vol. 30, no. 11, p. 1767.
Kang, L.-C., Chen, X., Chen, X.-Y., et al., Inorg. Chem. Commun., 2010, vol. 13, no. 1, p. 109.
Pogány, L., Moncol, J., Gál, M., et al., Inorg. Chim. Acta, 2017, vol. 462, p. 23.
Jayamani, A., Sethupathi, M., Ojwach, S.O., et al., Inorg. Chem. Commun., 2017, vol. 84, p. 144.
Ray, A., Sadhukhan, D., Rosair, G.M., et al., Polyhedron, 2009, vol. 28, no. 16, p. 3542.
Satapathi, S., Chattopadhyay, S., Bhar, K., et al., Inorg. Chem. Commun., 2011, vol. 14, no. 5, p. 632.
Sarkar, B., Drew, M.G.B., Estrader, M., et al., Polyhedron, 2008, vol. 27, no. 12, p. 2625.
Cai, L.F., Russ. J. Coord. Chem., 2017, vol. 43, no. 10, p. 663. https://doi.org/10.1134/S1070328417100025
Sun, W.H., Li, K.H., Liu, H., et al., Russ. J. Coord. Chem., 2017, vol. 43, no. 10, p. 693. https://doi.org/10.1134/S1070328417100104
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Rights and permissions
About this article
Cite this article
Wang, F.M., Li, L.J., Zang, G.W. et al. Syntheses, Crystal Structures, and Antimicrobial Activities of Copper(II) Complexes Derived from N,N'-Bis(5-Fluorosalicylidene)-1,3-Propanediamine. Russ J Coord Chem 47, 225–233 (2021). https://doi.org/10.1134/S1070328421030076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328421030076