Abstract
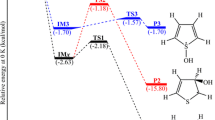
The potential energy surface (PES) and mechanism of the reaction of allyl acetate (AAC) with O3 are investigated by using density functional theory (DFT) and ab initio (MP2 and CCSD(T)) methods. The kinetics and main product branching ratios over the temperature range of (200–2,000 K) and at various pressures are obtained by employing multichannel Rice–Ramsperger–Kassel–Marcus (RRKM) theory. The results show that the main products are 2-oxoethyl acetate and formaldehyde. Two channels are found for the decomposition of primary ozonides: one path is corresponding to the 2-oxoethyl acetate + CH2OO formation (R1); the other channel products formaldehyde + CH3C(O)OCH2CHOO (R2). In the whole temperature range, R1 is calculated to be preferable and its product yield accounts for 60–77% of the total while R2 is found to contribute 20–40% to the total product yield. The overall rate constants show pressure independence and strong positive temperature dependence.







Similar content being viewed by others
References
Graedel TE, Hawkins DT, Claxton LD (1986) Atmospheric chemical compounds: sources, occurrence, and bioassay. Academic Press, Orlando, FL
Ferrari C (1995) Ph. D., Universite′ Joseph Fourier-Grenoble 1
Auerbach SS, Mahler J, Travlos GS, Irwin RD (2008) Toxicology 253:79–88
Picquet-Varrault B, Doussin JF, Durand-Jolibois R, Pirali O, Carlier P (2002) Environ Sci Technol 36:4081–4086
Calvé SL, Mellouki A, Bras GL, Treacy J, Wenger J, Sidebottom H (2000) J Atmos Chem 37:161–172
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2009) Environ Sci Pollut Res 16:641–648
Gonzalez C, Schlegel HBJ (1989) Chem Phys 90:2154–2161
Gonzalez C, Schlegel HBJ (1990) J Phys Chem 94:5523–5527
Zhang D, Zhang R (2005) J Chem Phys 122:114308-1–114308-12
Zhang D, Zhang RJ (2002) Am Chem Soc 124:2692–2703
Zhang D, Zhang RY, Park J, North SWJ (2002) Am Chem Soc 124:9600–9605
Jiang L, Xu YS, Ding AZJ (2010) Phys Chem A 114:12452–12461
Frisch MJ, Trucks GW, Schlegel HB, Gill PWM, Johnson BG, Robb MA, Cheeseman JR, Keith TA, Petersson GA, Montgomery JA, Raghavachari K, Allaham MA, Zakrzewski VG, Ortiz JV, Foresman JB, Cioslowski J, Stefanov BB, Nanayakkara A, Challacombe M, Peng CY, Ayala PY, Chen W, Wong MW, Andres JL, Replogle ES, Gomperts R Martin RL, Fox DJ Binkley JS, Defrees DJ, Baker J, Stewart JP, Head-Gordon M, Gonzales C, Pople JA (2003) Gaussian 03. Gaussian, Inc., Wallingford, CT
Hou H, Wang BJ (2007) Chem Phys 127:054306
Wang F, Sun H, Sun J, Jia X, Zhang Y, Tang Y, Pan X, Su Z, Hao L, Wang RJ (2010) Phys Chem A 114:3516–3522
Wang BS, Gu YS, Kong FNJ (1999) Phys Chem A 103:2060
Hou H, Li AX, Hu HY, Li YZ, Li H, Wang BSJ (2005) Chem Phys 122:224304
IUPAC (2006) Subcommittee on gas kinetic data evaluation. http//www.iupac-kinetic.ch.cam.ac.uk
Johnston HS, Heicklen JJ (1962) Phys Chem 66:532–533
Stein SE, Rabinovitch BSJ (1973) Chem Phys 58:2438
Astholz DC, Troe J, Wieters WJ (1979) Chem Phys 70:5107
Troe JJ (1977) Chem Phys 66:4745
Long XP, He B, Jiang XH, Wu X (2003) Chin J High Press Phys 17:247–254
Acknowledgments
This work was supported financially by the National Nature Science Foundation of China (NSFC Nos. 21077067, 20877049, 20737001). Independent Innovation Foundation of Shandong University (IIFSDU, project No. 2009JC016).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, M., Sun, Y., Cao, H. et al. Theoretical study of the ozonolysis of allyl acetate: mechanism and kinetics. Struct Chem 23, 201–208 (2012). https://doi.org/10.1007/s11224-011-9866-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9866-0