Abstract
Background, aim, and scope
Unsaturated esters are emitted to the atmosphere from biogenic and anthropogenic sources, including those from the polymer industry. Little information exists concerning the atmospheric degradation of unsaturated esters, which are mainly initiated by OH radicals. Limited information is available on the degradation of alkenes by Cl atoms and almost no data exists for the reactions of unsaturated esters with Cl atoms. This data is necessary to assess the impact of such reactions in maritime environments where, under circumstances, OH radical- and Cl atom-initiated oxidation of the compounds can be important. Rate coefficients for the reactions of chlorine atoms with vinyl acetate, allyl acetate, and n-butyl acrylate have been determined at 298 ± 3 K and atmospheric pressure. The kinetic data have been used in combination with that for structurally similar compounds to infer the kinetic contributions from the possible reaction channels to the overall reaction rate.
Materials and methods
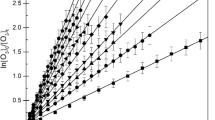
The decay of the organics was followed using in situ Fourier transform infrared spectroscopy and the rate coefficients were determined using a relative kinetic method and different hydrocarbon reference compounds.
Results
The following room temperature rate coefficients (in cm3 molecule−1 s−1) were obtained: k 1 (Cl + CH3C(O)OCH=CH2) = (2.68 ± 0.91) × 10−10, k 2 (Cl + CH3C(O)OCH2CH=CH2) = (1.30 ± 0.45) × 10−10, and k 3 (Cl + CH2=CHC(O)O(CH2)3CH3) = (2.50 ± 0.78) × 10−10, where the uncertainties are a combination of the 2σ statistical errors from linear regression analyses and a contribution to cover uncertainties in the rate coefficients of the reference hydrocarbons.
Discussion
This is the first kinetic study of the title reactions under atmospheric conditions. The kinetic data were analyzed in terms of reactivity trends and used to estimate the atmospheric lifetimes of the esters and assess their potential importance in the marine atmosphere.
Conclusions
Although reaction with OH radicals is the major atmospheric sink for the unsaturated esters studied, reaction with Cl atoms can compete in the early morning hours in coastal areas where the Cl concentration can reach peak values as high as 1 × 105 atoms cm−3. The calculated residence times show that the chemistry of unsaturated esters will impact air quality locally near their emission sources.
Recommendations and perspectives
The reactions need to be studied over the range of temperatures and pressures generally encountered in the marine atmosphere. In addition, product studies should also be performed as a function of temperature since this will allow degradation mechanisms to be derived, which are representative for the wide range of conditions occurring in marine environments. Inclusion of the kinetic and product data in tropospheric numerical models will allow an assessment of potential environmental impacts of the esters for different marine pollution scenarios.



Similar content being viewed by others
References
Aranda A, Martínez E, Díaz de Mera Y, Rodríguez A, Rodríguez D, Cuartero J (2003) Low-pressure study of the reactions of Cl atoms with acrylic acid and allyl alcohol. Atmos Environ 37:4361–4369
Barnes I, Becker KH, Mihalopoulos N (1994) An FTIR product study of the photooxidation of dimethyl disulfide. J Atm Chem 18:267–289
Blanco MB, Teruel MA (2008) Photodegradation of butyl acrylate in the troposphere by OH radicals: kinetics and fate of 1,2-hydroxyalcoxy radicals. J Phys Org Chem 21:397–401
Blanco MB, Taccone RA, Lane SI, Teruel MA (2006) On the OH-initiated degradation of methacrylates in the troposphere: gas-phase kinetics and formation of pyruvate. Chem Phys Lett 429(4–6):389–394
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel M (2008) Tropospheric chemical degradation of methyl acrylate and butyl methacrylate initiated by chlorine atoms. Geophysical Research Abstracts, 10
Cuevas CA, Notario A, Martínez E, Albaladejo J (2005) Influence of temperature in the kinetics of the gas-phase reactions of a series of acetates with Cl atoms. Atmos Environ 39:5091–5099
Ezell MJ, Wang W, Ezell AA, Soskin G, Finlayson-Pitts BJ (2002) Kinetics of reactions of chlorine atoms with a series of alkenes at 1 atm and 298 K: structure and reactivity. Phys Chem Chem Phys 4:5813–5820
Ferrari C (1995) Mise en évidence de composés organiques volatils provenant de la combustion d'un biocarburant: le diester. Mesure des constantes de photodégradation de composés spécifiques émis. In: Thèse de doctorat, Université Joseph Fourier
Grosjean D, Williams EL (1992) Environmental persistence of organic compounds estimated from structure–reactivity and linear free-energy relationships. Unsaturated aliphatics. Atmos Environ Part A 26:1395–1405
Grosjean E, Grosjean D (1998) Rate constants for the gas-phase reaction of ozone with unsaturated oxygenates. Int J Chem Kinet 30:21–29
Hein R, Crutzen PJ, Heimann M (1997) An inverse modeling approach to investigate the global atmospheric methane cycle. Global Biogeochem Cycl 11:43–76
Helmig D, Muller J, Klein W (1989) Volatile organic substances in forest atmosphere. Chemosphere 19:1399–1412
Le Calve S, Mellouki A, Le Bras G, Treacy J, Wenger J, Sidebottom H (2000) Kinetic studies of OH and O3 reactions with allyl and isopropenyl acetate. J Atmos Chem 37:161–172
Logan JA (1985) Tropospheric ozone—seasonal behaviour, trends, and anthropogenic influence. J Geophys Res 90:463–482
Notario A, Le Bras G, Mellouki A (1998) Absolute rate constants for the reactions of Cl atoms with a series of esters. J Phys Chem A 102:3112–3117
Picquet-Varrault B, Doussin JF, Durand-Jolibois R, Pirali O, Carlier P (2002) Kinetic and mechanistic study of the atmospheric oxidation by OH radicals of allyl acetate. Environ Sci Technol 36:4081–4086
Ragains ML, Finlayson-Pitts BJ (1997) Kinetics and mechanism of the reaction of Cl atoms with 2-methyl-1,3-butadiene (isoprene) at 298 K. J Phys Chem A 101:1509–1517
Rodriguez A, Rodriguez D, Soto A, Notario A, Aranda A, Díaz-de-Mera Y, Bravo I (2007) Relative rate measurements of reaction of unsaturated alcohols with atomic chlorine as a function of temperature. Atm Environ 41:4693–4702
Shu Y, Atkinson R (1995) Atmospheric lifetimes and fates of a series of sesquiterpenes. J Geophys Res 100:7275–7282
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastidge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Observations of molecular chlorine in coastal air. Nature 394:353–356
Stutz J, Ezell MJ, Ezell AA, Finlayson-Pitts BJ (1998) Rate constants and kinetic isotope effects in the reactions of atomic chlorine with n-butane and simple alkenes at room temperature. J Phys Chem A 102:8510–8519
Teruel MA, Lane SI, Mellouki A, Solignac G, Le Bras G (2006) OH reaction rate constants and UV absorption cross-sections of unsaturated esters. Atmos Environ 40(20):3764–3772
Teruel MA, Blanco MB, Luque GR (2007) Atmospheric fate of acrylic acid and acrylonitrile: rate constants with Cl atoms and OH radicals in the gas phase. Atmos Environ 41(27):5769–5777
Wang W, Ezell MJ, Ezell AA, Soskin G, Finlayson-Pitts BJ (2002) Rate constants for the reactions of chlorine atoms with a series of unsaturated aldehydes and ketones at 298 K: structure and reactivity. Phys Chem Chem Phys 4:1824–1831
Wingenter OW, Kubo MK, Blake NJ, Smith TW, Blake DR, Rowland FS (1996) Hydrocarbon and halocarbon measurements as photochemical and dynamical indicators of atmospheric hydroxyl, atomic chlorine and vertical mixing obtained during Lagrangian flights. J Geophys Res 101:4331–4340
Acknowledgements
The authors wish to acknowledge DAAD–PROALAR (Germany), the Deutsche Forschungsgemeinschaft (DFG), the EU project EUROCHAMP, SECYT (Argentina), CONICET (Argentina), ANPCyT–FONCYT (Argentina), SECyT–UNC (Córdoba, Argentina), Fundación Antorchas (Argentina), TWAS (Italy), and RSC (UK) for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Rights and permissions
About this article
Cite this article
Blanco, M.B., Bejan, I., Barnes, I. et al. The Cl-initiated oxidation of CH3C(O)OCH=CH2, CH3C(O)OCH2CH=CH2, and CH2=CHC(O)O(CH2)3CH3 in the troposphere. Environ Sci Pollut Res 16, 641–648 (2009). https://doi.org/10.1007/s11356-008-0096-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-008-0096-y