Abstract
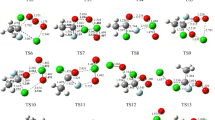
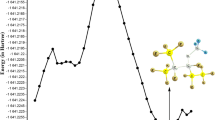
The singlet and triplet potential energy surfaces of the HO2 with CF2ClO2 reaction have been probed at the BMC-CCSD/cc-pVTZ level according to the B3LYP/6-311++G(d,p) level obtained geometrical structure. On the singlet PES, the association/dissociation, direct H- abstraction, and SN2 displacement mechanisms have been taken into account. On the triplet PES, SN2 displacement and indirect H- abstraction reaction mechanisms have been investigated and the H- abstraction channel makes more contribution to the CF2ClO2 with HO2 reaction. The rate constants have been computed at 10−10 to 1010 atm and 200–3000 K by RRKM-TST theory. The results show that at T ≤ 600 K, the generation of IM1 (CF2ClO4H) by collisional deactivation is dominant pathway; at high temperatures, the production of P8 (CF2ClOOH + O2(3Σ)) becomes predominate. The predicted data for CF2ClO2 + HO2 agrees closely with available experimental value. Moreover, OH radicals act as inhibitors in the CF2ClOOH→CF2O + HOCl and CF2ClOOH→CFClO + HOF reactions. The dominant products for the reaction of CF2ClOOH + OH are CF2ClO2 + H2O.







Similar content being viewed by others
References
Alicke B, Platt U, Stutz J (2002) Impact of nitrous acid photolysis on the total hydroxyl radical budget during the Limitation of Oxidant Production/Pianura Padana Produzione di Ozono Study In Milan. J Geophys Res Atmos 107:LOP 9–LOP 1
Anglada JM, Olivella S, Sole A (2006) Mechanistic study of the CH3O2• + HO2• → CH3O2H + O2 reaction in the gas phase. Computational evidence for the formation of a hydrogen-bonded diradical complex. J Phys Chem A 110:6073–6082
Aumont B, Chervier F, Laval S (2003) Contribution of HONO sources to the NOx/HOx/O3 chemistry in the polluted boundary layer. Atmos Environ 37:487–498
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Berndt T, Scholz W, Mentler B, Fischer L, Herrmann H, Kulmala M, Hansel A (2018) Accretion product formation from self- and cross-reactions of RO2 radicals in the atmosphere. Angew Chem Int Ed 57:3820–3824
Biggs P, Canosa-Mas CE, Shallcross DE, Vipond A, Wayne RP (1997) Kinetics of the reactions of CF3O2 with OH, HO2 and H. J Chem Soc Faraday Trans 93:2702–2705
Catoire V, Lesclaux R, Lightfoot PD, Rayez M-T (1994) Kinetic study of the reactions of CH2ClO2 with itself and with HO2 and theoretical study of the reactions of CH2ClO, between 251 and 600 K. J Phys Chem 98:2889–2898
Catoire V, Lesclaux R, Schneider WF, Wallington TJ (1996) Kinetics and mechanisms of the self-reactions of CCl3O2 and CHCl2O2 radicals and their reactions with HO2. J Phys Chem 100:14356–14371
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J Comput Chem 14:294–301
Finlayson-Pitts B, Pitts JN Jr (1999) Chemistry of the upper and lower atmosphere. Academic Press, New York
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09. Gaussian Inc., Wallingford, CT
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path. J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Hayman GD, Jenkin ME, Murrells TP, Johnson CE (1994) Tropospheric degradation chemistry of HCFC-123 (CF3CHCl2): a proposed replacement chlorofluorocarbon. Atmos Environ 28:421–437
Hayman GD, Battin-Leclerc F (1995) Kinetics of the reactions of the HO2 radical with peroxyl radicals derived from hydrochlorofluorocarbons and hydrofluorocarbons. J Chem Soc Faraday Trans 91:1313–1323
Holbrook KA, Pilling MJ, Robertson SH (1996) Unimolecular reactions; J. Wiley, Chichester, UK
Hossaini R, Chipperfield MP, Monge-Sanz BM, Richards NAD, Atlas E, Blake DR (2010) Bromoform and dibromomethane in the tropics: a 3-D model study of chemistry and transport. Atmos Chem Phys 10:719–735
Hou H, Wang BS (2007) Ab initio study of the reaction of propionyl (C2H5CO) radical with oxygen (O2). J Chem Phys 127:054306
Hou H, Wang BS, Gu YS (2000) Ab initio mechanism and multichannel RRKM-TST rate constant for the reaction of Cl(2P) with CH2CO (Ketene). J Phys Chem A 104:320–328
Hou H, Deng LZ, Li JC, Wang BS (2005) A systematic computational study of the reactions of HO2 with RO2: The HO2 + CH2ClO2, CHCl2O2, and CCl3O2 reactions. J Phys Chem A 109:9299–9309
Johnson D, Price DW, Marston G (2004) Correlation-type structure activity relationships for the kinetics of gas-phase RO2 self-reactions and reaction with HO2. Atmos Environ 38:1447–1458
Kleffmann J, Gavriloaiei T, Hofzumahaus A, Holland F, Koppmann R, Rupp L, Schlosser E, Siese M, Wahner A (2005) Daytime formation of nitrous acid: a major source of OH radicals in a forest. Geophys Res Lett 32:L05818
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys Rev B 37:785–789
Lesclaux R (1997) Combination of peroxyl radicals in the gas-phase. In: Alfassi ZB (ed) Peroxyl Radicals. Wiley, New York
Li MZ, Karu E, Brenninkmeijer C, Fischer H, Lelieveld J, Williams J (2018) Tropospheric OH and stratospheric OH and Cl concentrations determined from CH4, CH3Cl, and SF6 measurements. npj Clim Atmos Sci 1:29
Lin JM, Chao W (2017) Structure-dependent reactivity of Criegee intermediates studied with spectroscopic methods. Chem Soc Rev 46:7483–7497
Lynch BJ, Zhao Y, Truhlar DG (2005) The 6-31B(d) Basis set and the BMC-QCISD and BMC-CCSD multicoefficient correlation methods. J Phys Chem A 109:1643–1649
Maricq MM, Szente JJ, Hurley MD, Wallington TJ (1994) Atmospheric chemistry of HFC-134a: kinetic and mechanistic study of the CF3CFHO2 + HO2 reaction. J Phys Chem 98:8962–8970
McGivern WS, Kim HJ, Francisco JS, North SW (2004) Investigation of the atmospheric oxidation pathways of bromoform and dibromomethane: initiation via UV photolysis and hydrogen abstraction. J Phys Chem A 108:7247–7252
Mousavipour SH, Ramazani S, Shahkolahi Z (2009) Multichannel RRKM-TST and direct-dynamics VTST study of the reaction of hydroxyl radical with furan. J PhysChem A 113:2838–2846
Pratt DA, Tallman KA, Porter NA (2011) Free radical oxidation of polyunsaturated lipids: new mechanistic insights and the development of peroxyl radical clocks. Acc Chem Res 44:458–467
Ralph DG, Wayne RP (1982) Reactions of the chlorodifluoromethyl radical formed in the photolysis of halogenocarbon + ozone mixtures. J Chem Soc Faraday Trans 2(78):1815–1823
Sehested J, Nielsen OJ, Wallington TJ (1993) Absolute rate constants for the reaction of NO with a series of peroxy radicals in the gas phase at 295 K. Chem Phys Lett 213:457–464
Sehested J, Mogelberg T, Fagerstrom K, Mahmoud G, Wallington TJ (1997) Absolute rate constants for the self reactions of HO2, CF3CFHO2, and CF3O2 radicals and the cross reactions of HO2 with FO2, HO2 with CF3CFHO2, and HO2 with CF3O2 at 295 K. Int J Chem Kinet 29:673–682
Sun JY, Wang RS, Wang BS (2011) Theoretical study on the gas phase reaction of acrylonitrile with a hydroxyl radical. Phys Chem Chem Phys 13:16585–16595
Tang YZ, Sun JY, Zhang YJ, Wang RS (2014) The atmospheric degradation pathways of BrCH2O2: computational calculation on mechanisms of the reaction with HO2. Chemosphere. 111:545–553
Tang YZ, Lu CG, Sun JY, Shao YX, Gao Y, Fu ZH (2019) Computational investigations on the HO2 + CHBr2O2 reaction: mechanisms, products, and atmospheric implications. Environ Sci Pollut R 26:2345–2352
Villenave E, Lesclaux R (1995) The UV absorption spectra of CH2Br and CH2BrO2 and the reaction kinetics of CH2BrO2 with itself and with HO2 at 298 K. Chem Phys Lett 236:376–384
Wallington TJ, Nielsen OJ (1997) Peroxy radicals and the atmosphere. In: Alfassi ZB (ed) Peroxyl Radicals. Wiley, New York
Wallington TJ, Dagaut P, Kurylo MJ (1992) Ultraviolet absorption cross sections and reaction kinetics and mechanisms for peroxy radicals in the gas phase. Chem Rev 92:667–710
Wallington TJ, Hurley MD, Schneider WF, Sehested J, Nielsen OJ (1994) Mechanistic study of the gas-phase reaction of CH2FO2 radicals with HO2. Chem Phys Lett 218:34–42
Wayne RP (2000) Chemistry of Atmospheres, 3rd edn. Oxford University Press, Oxford
Wei WM, Zheng RH (2007) Theoretical study on the reaction mechanism of CH2ClO2 with HO2. J Mol Struct THEOCHEM 812:1–11
Wu F, Carr RW (1991) An investigation of temperature and pressure dependence of the reaction of CF2ClO2 radicals with nitrogen dioxide by flash photolysis and time resolved mass spectrometry. Int J Chem Kinet 23:701–715
Zhang WC, Du BN (2007) Ab initio quantum chemical studies of the reactions of CF3CFHO2 with HO2. Int J Quantum Chem 107:46–55
Zhang YJ, Sun JY, Chao K, Sun H, Wang F, Tang SW, Pan XM, Zhang JP, Wang RS (2012) Mechanistic and kinetic study of CF3CH=CH2 + OH reaction. J Phys Chem A 116:3172–3181
Zhang YJ, Chao K, Sun JY, Su ZM, Pan XM, Zhang JP, Wang RS (2013) Theoretical study on the gas phase reaction of allyl alcohol with hydroxyl radical. J Phys Chem A 117:6629–6640
Funding
This work was supported by the Natural Science Foundations of China (No. 21707062).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., He, B., Wang, Z. et al. Atmospheric chemistry of CF2ClO2: a theoretical study on mechanisms and kinetics of the CF2ClO2 + HO2 reaction. Environ Sci Pollut Res 27, 33965–33974 (2020). https://doi.org/10.1007/s11356-020-09580-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09580-9