Abstract
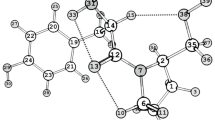
l-Tyrosine alkyl esters are used as prodrugs for l-tyrosine. Although prodrugs are often designed for their behavior in solution, understanding their solid-state properties is the first step in mastering drug delivery. The crystal structure of l-tyrosine methyl ester has been determined and compared to published structures of l-tyrosine and its ethyl and n-butyl esters. It is almost isostructural with the other esters: it crystallizes in the orthorhombic chiral space group P212121, a = 5.7634(15) Å, b = 12.111(2) Å, c = 14.3713(19) Å, V = 1003.1(4) Å3 with Z′ = 1. Their main packing motif is a C(9) infinite hydrogen-bond chain, but the conformation of l-tyrosine methyl ester is different from the other two: eclipsed versus U-shaped, respectively. The published structure of the ethyl ester, which was incomplete, has been confirmed by X-ray powder diffraction data. Because l-tyrosine methyl ester is very stable (28 years stored at room temperature), and its hydrolysis rate is relatively low, it should be one of the better prodrugs among the alkyl esters of tyrosine.









Similar content being viewed by others
Notes
The absolute configuration of TME was considered l, since this was the configuration of the original tyrosine used for the synthesis.
References
Oliyai R, Stella VJ (1993) Annu Rev Pharmacol Toxicol 32:521–544
Mndzhoyan OL, Agadzhanyan TE, Fradkina NN, Avakyan OM, Noravyan OS (1971) Pharm Chem J 5:383–385
Kawabata A, Kasamatsu K, Takagi H (1993) Eur J Pharmacol 233:255–260
Steffansen B, Mørk N, Bundgaard H (1989) Acta Pharm Nord 2:47–56
Kumagai H, Echigo T, Hideyuki S, Tochikura T (1989) Agric Biol Chem 53:1429–1430
Carlsson PAE, Corrodi HR (1975) Aktiebolaget Hassle, Treatment of Parkinson’s disease. US Patent 19730820
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzucka N, Mandel R, Björklung A (2002) J Neurosci 22:2780–2791
Roeske RW (1959) Chem Ind (London) 1121–1122
Kahns AH, Buur A, Bundgaard H (1993) Pharm Res 10:68–74
Huang CH, Kimura R, Bawarshi-Nassar R, Hussain A (1985) J Pharm Sci 74:1298
Chien YW, Su KSE, Chang S-F (1989) Drugs Pharmaceut Sci 39:49–53
McDonald CE, Balls AK (1956) J Biol Chem 221:993–1003
Glazer AN (1966) J Biol Chem 241:635–638
Khawas B, Murti GSRK (1969) Acta Crystallogr Sect B 25:1006–1009
Mostad A, Nissen HM, Romming C (1972) Acta Chem Scand 26:3819–3833
Boggs R, Donohue J (1971) Acta Crystallogr Sect B 27:247
Frey MN, Koezle TF, Lehmann MS, Hamilton WC (1973) J Chem Phys 58:2547
Srinivasan R (1959) Proc Indian Acad Sci A 50:19–50
Srinivasan R (1959) Proc Indian Acad Sci A 49:340–373
Khawas B (1970) Acta Crystallogr Sect B 26:1919–1922
Mostad A, Romming C (1973) Acta Chem Scand 27:401–410
Mostad A, Nissen HM, Romming C (1971) Acta Chem Scand 25:1145
Mostad A, Nissen HM, Romming C (1971) Tetrahedron Lett 2131
Khawas B (1986) J Appl Crystallogr 19:410
Leahey A, Olmstead MM (2001) Crystal structure of DL-tyrosine: CCDC 168369
Pieret AF, Durant F, Griffé M, Germain G, Debaerdemaeker T (1970) Acta Crystallogr Sect B 26:2117
Qian SH, Zhu HL, Tiekink ERT (2006) Acta Crystallogr Sect E 62:o882–o884
Bryndal I, Jaremko M, Jaremko L, Lis T (2006) Acta Crystallogr Sect C 62:o111–o114
Enraf Nonius (1994) CAD4 Express Software Enraf Nonius, Delft, The Netherlands
Harms K, Wocadlo S (1995) XCAD-CAD4 Data reduction University of Marburg, Marburg
Sheldrick GM (2008) Acta Crystallogr Sect A 64:112–122
Spek AL (1990) Acta Crystallogr Sect A 46:C-34
Ballon J, Comparat V, Pouxe J (1983) Nucl Instrum Methods Phys Res Sect A 217:213–216
Moggach SA, Allan DR, Parsons S, Sawyer (2006) Acta Crystallogr Sect B 62:310–320
Fabbiani PA, Byrne LT, McKinnon JJ, Spackman MA (2007) CrystEngComm 9:728–731
McKinnon JJ, Fabbiani FPA, Spackman MA (2007) Cryst Growth Des 7:755–769
Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ (2009) J Pharm Sci 98:2010–2026
Martins FT, Paparidis N, Doriguetto AC, Ellena J (2009) Cryst Growth Des 9:5283–5292
Martins FT, Bocelli MD, Bonfilio R, de Araujo MB, de Lima PV, Neves PP, Veloso MP, Ellena J, Doriguetto AC (2009) Cryst Growth Des 9:3235–3244
Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ (2009) Cryst Growth Des 9:1054–1065
Bathori NB, Bourne SA (2009) J Chem Crystallogr 39:539–543
McIldowie MJ, Gandy MN, Skelton BW, Brotchie JM, Koutsantonis GA, Spackman MA, Piggott MJ (2010) J Pharm Sci 99:234–245
Wolff SK, Grimwood DJ, McKinnon JJ, Jayatilaka D, Spackman MA (2007) Crystal Explorer 2.1 http://hirshfeldsurfacenet.blogspot.com/
Jayatilaka D, Grimwood DJ, Lee A, Lemay A, Russel AJ, Taylor C, Wolff SK (2006) TONTO: a system for computational chemistry
Spackman MA, Jayatilaka D (2009) CrystEngComm 11:19–32
Spackman MA, McKinnon JJ, Jayatilaka D (2008) CrystEngComm 10:377–388
Spackman MA, McKinnon JJ (2002) CrystEngComm. 4:378–392
Braun DE, Gelbrich T, Jetti RKR, Kahlenberg V, Price SL, Griesser UJ (2008) Cryst Growth Des 8:1977–1989
Braun DE, Gelbrich T, Kahlenberg V, Laus G, Wieser J, Griesser UJ (2008) New J Chem 32:1677–1685
Negrier P, Pardo LC, Salud J, Tamarit JL, Barrio M, Lopez DO, Wurflinger A, Mondieig D (2002) Chem Mater 14:1921
Parat B, Pardo LC, Barrio M, Tamarit JL, Negrier P, Salud J, Lopez DO, Mondieig D (2005) Chem Mater 17:3359–3365
Fortes AD, Wood IG, Knight KS (2008) Phys Chem Miner 35:207
Negrier P, Barrio M, Tamarit JL, Veglio N, Mondieig D (2010) Cryst Growth Des 10:2793–2800
Authier A (2003) International tables for Crystallography, D: physical properties of crystals, section 1.4, 1st edn. Wiley, London
Belousov RI, Filatov SK (2007) Glass Phys Chem 33:271–275
Etter MC (1990) Acc Chem Res 23:120–126
Connolly ML (1983) J Appl Crystallogr 16:548–558
Kitaigorodsky AI (1973) Molecular crystals and molecules. Academic Press, New York
Mooibroek TJ, Gamez P, Reedijk J (2008) CrystEngComm 10:1501–1515
Helluy X, Sebald A (2003) J Phys Chem B 107:3290–3296
Kaminski W (2004) WinTensor (http://www.wintensor.com) version 1.1
Acknowledgments
The authors thank Philippe Bénas (Université Paris Descartes) and M. Marsal (Universitat Politècnica de Catalunya) for assistance with single-crystal X-ray diffraction data collection and SEM examinations, respectively. The authors thank the late Nestor Veglio for the powder diffraction data collection. Part of this study was supported by the Spanish grant FIS2008-00837 and by the Catalan government (2008SGR-1251). RC thanks the Generalitat de Catalunya (2007PIV00011) for an invited position at the Universitat Politècnica de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nicolaï, B., Mahé, N., Céolin, R. et al. Tyrosine alkyl esters as prodrug: the structure and intermolecular interactions of l-tyrosine methyl ester compared to l-tyrosine and its ethyl and n-butyl esters. Struct Chem 22, 649–659 (2011). https://doi.org/10.1007/s11224-010-9723-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9723-6