Abstract
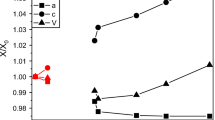
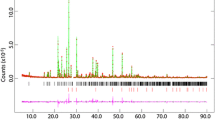
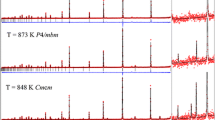
We have collected high-resolution neutron powder diffraction patterns from MgSO4·11D2O over the temperature range 4.2–250 K. The crystal is triclinic, space-group \( \text{P} \bar{1} \) (Z = 2) with a = 6.72746(6) Å, b = 6.78141(6) Å, c = 17.31803(13) Å, α = 88.2062(6)°, β = 89.4473(8)°, γ = 62.6075(5)°, and V = 701.140(6) Å3 at 4.2 K, and a = 6.75081(3) Å, b = 6.81463(3) Å, c = 17.29241(6) Å, α = 88.1183(3)°, β = 89.4808(3)°, γ = 62.6891(3)°, and V = 706.450(3) Å3 at 250 K. Structures were refined to wRp = 3.99 and 2.84% at 4.2 and 250 K, respectively. The temperature dependence of the lattice parameters over the intervening range have been fitted with a modified Einstein oscillator model which was used to obtain the coefficients of the thermal expansion tensor. The volume thermal expansion, αV, is considerably smaller than ice Ih at all temperatures, and smaller even than MgSO4·7D2O (although ∂αV/∂T is very similar for both sulfates); MgSO4·11D2O exhibits negative αV below 55 K (compared to 70 K in D2O ice Ih and 20 K in MgSO4·7D2O) The relationship between the magnitude and orientation of the principal axes of the expansion tensor and the main structural elements are discussed.












Similar content being viewed by others
Notes
At the time of the neutron study (November 2004) it was still thought that Fritzsche’s salt was a dodecahydrate and we prepared our sample accordingly; as a result, our sample consisted of MgSO4·11D2O + water ice.
References
Ashcroft NW, Mermin ND (1976) Solid state physics. Harcourt Brace College Publishers, Orlando
Batsanov AS (2000) Magnesium sulfate hexahydrate at 120 K. Acta Cryst C 56:e230–e231. doi:10.1107/S0108270100006442
Baur WH (1964) On the crystal chemistry of salt hydrates. II. A neutron diffraction study of MgSO4·4H2O. Acta Cryst 17:863–869. doi:10.1107/S0365110X64002304
Baur WH, Rolin JL (1972) Salt hydrates. IX. The comparison of the crystal structure of magnesium sulfate pentahydrate with copper sulfate pentahydrate and magnesium chromate pentahydrate. Acta Cryst B 28:1448–1455. doi:10.1107/S0567740872004443
Brand HEA, Fortes AD, Wood IG, Alfredsson M, Vočadlo L (2006) High-pressure properties of planetary sulfate hydrates determined from interatomic potential calculations. Lunar Planet Sci Conf 37, abstract #1310. http://www.lpi.usra.edu/meetings/lpsc2006/pdf/1310.pdf
Cardoso GM (1930) Los modernos métodos roentgenográficos aplicados en la determinación de la estuctura cristalina de la epsomita. Trabajos del Museo Nacional de Ciencias naturales. Serie Geol 37:5–133
Chiari G, Ferraris G (1982) The water molecule in crystalline hydrates studied by neutron diffraction. Acta Cryst B 38:2331–2341. doi:10.1107/S0567740882008747
Chipera SJ, Vaniman DT, Bish DL, Carey JW, Feldman WC (2005) Experimental stability and transformation kinetics of magnesium sulfate hydrates that may be present on Mars. Lunar Planet Sci Conf 36, abstract #1497. http://www.lpi.usra.edu/meetings/lpsc2005/pdf/1497.pdf
Chou I, Seal RR (2007) Magnesium and calcium sulfate stabilities and the water water budget on Mars. J Geophys Res Planets 112, article E11004. doi:10.1029/2007JE002898
Cruickshank DW (1961) The rôle of 3d-orbitals in π-bonds between (a) silicon, phosphorus, sulphur, or chlorine and (b) oxygen and nitrogen. J Chem Soc 1961:5486–5504. doi:10.1039/JR9610005486
Dalton III JB (2003) Spectral behaviour of hydrated sulfate salts: implications for Europa mission spectrometer design. Astrobiology 3:771–784. doi:10.1089/153110703322736097
Dalton III JB, Prieto-Ballesteros O, Kargel JS, Jamieson CS, Jolivet J, Quinn R (2005) Spectral comparison of heavily hydrated salts with disrupted terrains on Europa. Icarus 177(2):472–490. doi:10.1016/j.icarus.2005.02.023
Day S, Asphaug E, Bruesch L (2002) Cumulates, dykes and pressure solution in the ice-salt mantle of Europa. EOS, Transactions of the American Geophysical Union, vol 84, no 46, Fall Meeting Supplement, Abstract P72B–0507
Dougherty AJ, Hogenboom DL, Kargel JS (2007) Volumetric and optical studies of high pressure phases of MgSO4–H2O with applications to Europa. Lunar Planet Sci Conf 38, abstract #2275. http://www.lpi.usra.edu/meetings/lpsc2007/pdf/2275.pdf
Ferraris G, Jones DW, Yerkess J (1973) Refinement of the crystal structure of magnesium sulphate heptahydrate (Epsomite) by neutron diffraction. J Chem Soc Dalton Trans 1973:816–821. doi:10.1039/DT9730000816
Ferraris G, Ivaldi G (1984) X-OH and O-H···O bond lengths in protonated oxoanions. Acta Cryst B 40:1–6. doi:10.1107/S0108768184001671
Fialips CI, Carey JW, Vaniman DT, Feldman WC, Bish DL, Mellon MT (2004) Sub-surface deposits of hydrous silicates or hydrous magnesium sulfates as hydrogen reservoirs near the Martian equator: Plausible or not? Lunar Planet Sci Conf 35, abstract #2054. http://www.lpi.usra.edu/meetings/lpsc2004/pdf/2054.pdf
Finney JL (1995) The complimentary use of X-ray and neutron diffraction in the study of crystals. Acta Crystallogr Sect B: Struct Sci B 51:447–467. doi:10.1107/S0108768195002734
Fortes AD (2005) From Surrey to the moons of Jupiter (via Mars): the story of epsomite. Axis 1(9):1–28 (www.MineralogicalRecord.com)
Fortes AD, Wood IG, Knight KS, Brodholt JP, Alfredsson M, McGrady GS, Vočadlo L (2003) A high resolution neutron powder diffraction study of ammonia dihydrate (ND3·2D2O) phase I. J Chem Phys 119(20):10806–10813. doi:10.1063/1.1619371
Fortes AD, Wood IG, Grigoriev D, Alfredsson M, Kipfstuhl S, Knight KS, Smith RI (2004) No evidence of large-scale proton ordering in Antarctic ice from powder neutron diffraction. J Chem Phys 120(24):11376–11379. doi:10.1063/1.1765099
Fortes AD, Wood IG, Vočadlo L, Brand HEA, Grindrod PM, Joy KH, Tucker MG (2006a) The phase behaviour of epsomite (MgSO4·7H2O) to 50 kbar: planetary implications. Lunar Planet Sci Conf 37, abstract #1029. http://www.lpi.usra.edu/meetings/lpsc2006/pdf/1029.pdf
Fortes AD, Wood IG, Alfredsson M, Vočadlo L, Knight KS (2006b) The thermoelastic properties of MgSO4·7D2O (epsomite) from powder neutron diffraction and ab initio simulation. Eur J Min 18(4):449–462. doi:10.1127/0935–1221/2006/0018-0449
Fortes AD, Wood IG, Knight KS (2006c) Neutron powder diffraction studies of sulfuric acid hydrates. I: The structure of sulfuric acid hemitriskaidekahydrate, D2SO4·6½D2O. J Chem Phys 125(14), article 144510. doi:10.1063/1.2356860
Fortes AD, Grindrod PM, Trickett SK, Vočadlo L (2007a) Ammonium sulfate on Titan: possible origin and role in cryovolcanism. Icarus 188(1):139–153. doi:10.1016/j.icarus.2006.11.002
Fortes AD, Wood IG, Vočadlo L, Brand HEA, Knight KS (2007b) Crystal structures and thermal expansion of α-MgSO4 and β-MgSO4 from 4.2–300 K by neutron powder diffraction. J Appl Cryst 40(4). doi:10.1107/S0021889807029937 (in press)
Friedman HL, Lewis L (1976) The coordination geometry of water in some salt hydrates. J Sol Chem 5(7):445–455. doi:10.1007/BF00650462
Fritzsche CJ (1837) Ueber eine neue Verbindung der schwefelsauren Talkerde mit wasser. Bulletin scientifique publié par l’Académie Impériale des Sciences de St. Pétersbourg 2(13):193–196: Annalen der Physik und Chemie (Poggendorff’s Annalen) 42:577–580. http://www.gallica.bnf.fr/ark:/12148/bpt6k15127
Gärtner RS, Genceli FE, Trambitas DO, Witkamp GJ (2005) Impurity gradients in solution-grown ice and MgSO4·12H2O crystals measured by cryo-laser ablation and high-resolution-induced-coupled plasma mass spectrograph. J Cryst Growth 275:e1773–e1887. doi:10.1016/j.jcrystgro.2004.11.194
Grasset O, Mevel L, Mousis O, Sotin C (2001) The pressure dependence of the eutectic composition in the system MgSO4–H2O: implications for the deep liquid layer of icy satellites. Lunar Planet Sci Conf 31, abstract #1524. http://www.lpi.usra.edu/meetings/lpsc2001/pdf/1524.pdf
Hawthorne FC, Groat LA, Raudsepp M, Ercit TS (1987) Kieserite, Mg(SO4)(H2O), a titanite-group mineral. Neues Jarhb Mineral Abhand 157:1221–1232
Himawan C (2002) Recovery of magnesium sulfate and ice from magnesium sulfate industrial stream by eutectic freezing. In: Chemical engineering transactions, volume 1, Proceedings of the 15th international symposium on industrial crystallisation, pp 951–956
Hodenberg RF von, Kühn R (1967) Zur Kenntnis der magnesiumsulfathydrate und der effloreszenzen des Kieserits von Hartsalzen. Kali Steinsalz 4(10):326–340
Hogenboom DL, Kargel JS, Ganasan JP, Lee L (1995) Magnesium sulfate-water to 400 MPa using a novel piezometer: densities, phase equilibria, and planetological implications. Icarus 115(2):258–277. doi:10.1016/icar.1995.1096
Hogenboom DL, Kargel JS, Reiter ML, Khor YN (2002) Volume changes attending hydration of quenched magnesium sulfate brine—the tectonics of Ganymede’s sulci. Lunar Planet Sci Conf 33, abstract #1638. http://www.lpi.usra.edu/meetings/lpsc2002/pdf/1638.pdf
Ibberson RM, David WIF, Knight KS (1992) The High Resolution Neutron Powder Diffractometer (HRPD) at ISIS—a user guide. RAL-92–031. Rutherford Appleton Laboratory, Oxfordshire. http://www.isis.rl.ac.uk/crystallography/documentation/HRPDguide
Kaminski W (2004) WinTensor 1.1 (http://www.wintensor.com)
Kargel JS (1991) Brine volcanism and the interior structures of asteroids and icy satellites. Icarus 94(2):369–390. doi:10.1016/0019-1035(91)90235-L
Knight KS (1996) A neutron powder diffraction determination of the thermal expansion tensor of crocoite (PbCrO4) between 60 K and 290 K. Min Mag 60:963–972
Knight KS, Stretton IC, Schofield PF (1999) Temperature evolution between 50 K and 320 K of the thermal expansion tensor of gypsum derived from neutron powder diffraction data. Phys Chem Min 26:477–483. doi:10.1007/s002690050210
Larsen AC, von Dreele RB (2000) General Structure Analysis System (GSAS). Los Alamos National Laboratory Report LAUR 86–748, Los Alamos. http://www.ncnr.nist.gov/xtal/software/gsas.html
Louisnathan SJ, Hill RJ, Gibbs GV (1977) Tetrahedral bond length variations in sulfates. Phys Chem Min 1:53–69. doi:10.1007/BF00307979
McCarthy C, Cooper RF, Kirby SH, Rieck KD, Stern LA (2007) Solidification and microstructures of binary ice-I/hydrate eutectic aggregates. Am Min 92(10):1550–1560. doi:10.2138/am.2007.2435
McCord TB, Hansen GB, Matson DL, Johnson TV, Crowley JK, Fanale FP, Carlson RW, Smythe WD, Martin PD, Hibbitts CA, Granahan JC, Ocampo A, the NIMS Team (1999) Hydrated salt minerals on Europa’s surface from the Galileo Near-Infrared Mapping Spectrometer (NIMS) investigation. J Geophys Res Planets 104(E5):11827–11851. doi:10.1029/1999JE900005
McCord TB, Hansen GB, Hibbitts CA (2001) Hydrated salts on Ganymede’s surface: evidence of an ocean below. Science 292:1523–1525. doi:10.1126/science.1059916
Pauffler P, Weber T (1999) On the determination of linear thermal expansion cofficients of triclinic crystals using X-ray diffraction. Eur J Min 11(4):721–730
Peterson RC, Wang R (2006) Crystal molds on Mars: melting of a possible new mineral species to create Martian chaotic terrain. Geology 34(11):957–960. doi:10.1130/G22768A.1
Peterson RC, Nelson W, Madu B, Shurvell HF (2007) Meridianiite (MgSO4·11H2O): a new mineral species observed on Earth and predicted to exist on Mars. American Min 92(10):1756–1759. doi:10.2138/am.2007.2668
Polo M, Gérard N, Lallemont M (1971) Étude du système binaire MgSO4 - H2O. Comptes rendus hebdomadaires des séances de l’Académie des Sciences, Série C. Sci Chim 272:642–645
Röttger K, Endriss A, Ihringer J (1994) Lattice constants and thermal expansion of H2O and D2O ice Ih between 10 and 265 K. Acta Cryst Sect B Struct Sci 50:644–648. doi:10.1107/S0108768194004933
Schofield PF, Knight KS (2000) Neutron powder diffraction studies of the thermal behaviour of deuterated chalcanthite. Phys B 276–278:897–898. doi:10.1016/S0921-4526(99)01282-X
Schofield PF, Knight KS, van der Houwen JAM, Valsami-Jones E (2004) The role of hydrogen bonding in the thermal expansion and dehydration of brushite, di-calcium phosphate dihydrate. Phys Chem Min 31:606–624. doi:10.1007/s00269–004-0419-6
Vaniman DT, Bish DL, Chipera SJ, Carey JW (2004) Salt attack on rocks and expansion of soils on Mars. American Geophysical Union Fall Meeting, abstract P21A–0207
Van’t Hoff JH, Meyerhoffer W, Smith (1901) Untersuchungen über die bildungsverhältnisse der oceanischen salzablagerungen, insbesondere des Stassfurter salzlagen. XXIII. Das auftreten von Kieserit bei 25°. Sitzungsberichte der Preussichen Akademie der Wissenshaften, pp 1034–1044
Vočadlo L, Knight KS, Price GD, Wood IG (2002) Thermal expansion and crystal structure of FeSi between 4 and 1173 K determined by time-of-flight neutron powder diffraction. Phys Chem Min 29(2):132–139. doi:10.1007/s002690100202
Zalkin A, Ruben H, Templeton DH (1964) The crystal structure and hydrogen bonding of magnesium sulfate hexahydrate. Acta Cryst 17:235–240. doi:10.1107/S0365110X64000603
Acknowledgments
The authors wish to thank the STFC ISIS facility for beam time, and technical support staff for invaluable assistance. We are grateful to Ronald Peterson and Ruiyao Wang for sharing their structural data with us prior to publication. We also thank Christine McCarthy, David Hogenboom, and Andrew Dougherty for informative discussions about their work on MS11, and thank Lidunka Vočadlo for contributions to the data analysis. Giovanni Ferraris and one anonymous reviewer provided useful comments, which improved the manuscript. This work was funded by the Science and Technology Facilities Council (STFC), U. K., grant numbers PPA/P/S/2003/00247 and PP/E006515/1.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fortes, A.D., Wood, I.G. & Knight, K.S. The crystal structure and thermal expansion tensor of MgSO4–11D2O(meridianiite) determined by neutron powder diffraction. Phys Chem Minerals 35, 207–221 (2008). https://doi.org/10.1007/s00269-008-0214-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-008-0214-x