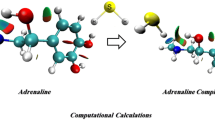
Abstract
In this study, complexes formed via hydrogen bond interactions between N-protonated adrenaline (AdH+) and DMSO have been studied by density functional theory (DFT). The relevant geometries, energies, and IR characteristics of the hydrogen bonds (H-bonds) have been systematically investigated. The natural bond orbital (NBO) and the quantum theory of atoms in molecule (QTAIM) analysis have also been applied to understand the nature of the hydrogen bonding interactions in complexes. The H-bonds involving amino or hydroxyls as H-donor are dominant H-bonds in complexes and are attributed to strong H-bonds. The weak H-bonds, such as π H-bonds and H-bonds involving methyl (DMSO) or methenyls (C2H6 and C5H7 of AdH+) as H-acceptors, were found in complexes as well. The complexes in which the dominant H-bond involves amino of AdH+ as H-donor are more stable than those with the dominant H-bond involving hydroxyls as H-donor. Some relationships between various properties of QTAIM, NBO, geometry as well as frequency were also investigated.





Similar content being viewed by others
References
El Bouhouti H, Naranjo-Rodriguez I, de Cisneros J, ElKaoutit M, Temsamani KR, Bouchta D, Aguilera LMC (2009) Talanta 79:22
Chernyshov DV, Shuedene NV, Antipova ER, Pletnev IV (2008) Anal Chim Acta 621:178
Song YZ (2007) Spectrochim Acta A 67:1169
Xue K-H, Liu J-M, Wei R-B, Chen S-P (2006) Chem Phys 327:319
Xie P, Chen X, Wang F, Hu C, Hu S (2006) Colloid Surf B 48:17
Salimi A, MamKhezri H, Hallaj R (2006) Talanta 70:823
Macleod NA, Simons JP (2004) Phys Chem Chem Phys 6:2821
Yu ZY, Guo DJ, Wang HQ (2004) Chin J Chem Phys 17:149
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford, UK
Popelier PLA (2000) Atoms in molecules: an introduction. Prentice Hall, London
Matta CFJBR (2007) The quantum theory of atoms in molecules: from solid state to DNA and drug design. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Reed AE, Weinhold F, Curtiss LA, Pochatko DJ (1986) J Chem Phys 84:5687
Beche AD (1988) Phys Rev A 38:3098
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650
McLean AD, Chandler GS (1980) J Chem Phys 72:5639
Maul R, Ortmann F, Preuss M, Hannewald K, Bechstedt F (2007) J Comput Chem 28:1817
Rozas I, Alkorta I, Elguero J (2008) Struct Chem 19:923
Tian SX (2004) J Phys Chem B 108:20388
Tian ZX, Pawlow A, Poutsma JC, Kass SR (2007) J Am Chem Soc 129:5403
van Mourik T (2004) Phys Chem Chem Phys 6:2827
Boys SF, Bernardi F (1970) Mol Phys 19:553
Koch U, Popelier PLA (1995) J Phys Chem 99:9747
Frisch MJ, Truchs GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2003) Gaussian, Inc., Pittsburgh, PA
Biegler-König F (2000) University of Applied Sciences. Bielefeld, Germany
Alagona G, Ghio C (2007) J Mol Struct Theochem 811:223
Carcabal P, Snoek LC, Van Mourik T (2005) Mol Phys 103:1633
Macleod NA, Simons JP (2006) Mol Phys 104:3317
Macleod NA, Simons JP (2007) Mol Phys 105:2
Popelier PLA (1998) J Phys Chem A 102:1873
Arnold WD, Oldfield E (2000) J Am Chem Soc 122:12835
Pacios LF (2004) J Phys Chem A 108:1177
Acknowledgment
This work is supported by Tianjin Science and Technology Development Fund Projects in Colleges and Universities (No. 20080504).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Z., Dai, Y. & Yu, L. Density functional theory and topological analysis on the hydrogen bonding interactions in N-protonated adrenaline–DMSO complexes. Struct Chem 21, 863–872 (2010). https://doi.org/10.1007/s11224-010-9621-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9621-y