Abstract
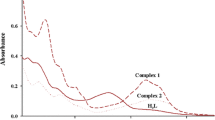
The ligand of 4-phenyl-1-(1-phenylethylidene)thiosemicarbazide (HL) and its metal complexes of ZnL2 (1) and CuL2 (2) have been synthesized. Elemental analysis, IR and X-ray single crystal diffraction characterizations for 1 and 2 have been carried out. In the complexes 1 and 2, the central metallic ions Zn2+ and Cu2+, coordinate with two deprotonated ligands, L−, respectively and adopt distorted tetrahedral geometries. The thermal analyses result shows that the two complexes undergo two similar decomposition processes because of their similar geometric configurations. For the two complexes, UV–Vis spectra have been measured and DFT calculations at B3LYP/LANL2DZ level of theory prove that the electronic spectra of 1 are corresponding with intraligand electronic transitions, and the electronic spectra of 2 are attributed to intraligand electronic transitions as well as d–d electronic transitions. Fluorescence spectra measurements indicate that the complex of 1 emits stronger fluorescence than the ligand of HL, and the complex 2 does not emit fluorescence at all. Electrochemical analyses reveal that both the oxidation peak currents and the reduction peak currents of 1 and 2 are stronger than those of the ligand, respectively.







Similar content being viewed by others
References
Latheef L, Manoj E, Kurup MRP (2007) Polyhedron 26:4107
West DX, Liberta AE, Padhye SB, Chikate RC, Sonawane PB, Kumbhar AS, Yerande RG (1993) Coord Chem Rev 123
Kasai K, Aoyagi M, Fujita M (2000) J Am Chem Soc 122:2140
Kitagawa S, Kitaura R, Noro SI (2004) Angew Chem Int Ed 43:2334
Hoshino N, Ito T, Nihei M, Oshio H (2003) Inorg Chem Commun 6:377
Khandar AA, Nejati K (2000) Polyhedron 19:607
Chen C, Huang D, Zhang X, Chen F, Zhu H, Liu Q, Zhang C, Liao D, Li L, Sun L (2003) Inorg Chem 42:3540
Gao EQ, Bai SQ, He Z, Yan CH (2005) Inorg Chem 44:677
Brooker S, Davidson TC (2000) Inorg Chim Acta 306:227
Adams H, Fenton DE, Ryan SJ (1999) Inorg Chem Commun 2:52
Zhou HP, Li DM, Wang P, Cheng LH, Gao YH, Zhu YM, Wu JY, Tian YP, Tao XT, Jiang MH, Fun HK (2007) J Mol Struct 826:205
Jian FF, Li Y, Xiao HL (2005) Acta Crystallogr Sect E 61:o2219
Sheldrick GM (1997) SHELXTL V5.1 software reference manual. Bruker AXS, Inc., Madison
Wilson AJ (1992) International table for X-ray crystallography, Kluwer, Dordrecht, The Netherlands; vol. C: Tables 6.1.1.4 (pp. 500–502) and 4.2.6.8 (pp. 219–222), respectively
Peng C, Ayals PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 49:17
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T Jr, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian Inc, Wallingford CT
Runge E, Gross EKU (1984) Phys Rev Lett 52:997
Petersilka M, Gossmann UJ, Gross EKU (1966) Phys Rev Lett 76:1212
Armelao L, Bandoli G, Barreca D, Bottaro G, Tondello E, Venzo A, Nittadini A (2007) Appl Organomet Chem 21:246
Liang H, Liu B, Hu RX, Yu KB (2000) J Indian Chem Soc 77:486
Richter R, Beyer L, Andrianov VG, Struchkov YT (1984) Z Anorg Allg Chem 513:123
Acknowledgments
This work was supported by Fund of Huanyin Normal University (07HSBS004, 08HSJSK003), Fund of Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials (JSKC08047, JSKC09065) and National Nature Science Foundation of China (No. 20671038).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Zhao, P., Shao, D. et al. Synthesis, characterization and spectra studies on Zn(II) and Cu(II) complexes with thiocarbamide ligand containing Schiff base group. Struct Chem 20, 995–1003 (2009). https://doi.org/10.1007/s11224-009-9502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9502-4