Abstract
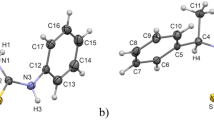
The first three examples of the cocrystallization of covalent diastereomers of phosphorus compounds containing different chiral elements (centres of chirality on carbon and phosphorus, and an axis of chirality) are reported. The phenomenon can be attributed to the complementarity of the molecular shapes of the diastereomers. The X-ray data of all the crystals exhibit centrosymmetric statistics regardless of their very different natures.











Similar content being viewed by others
References
Fogassy E, Nogradi M, Kozma D, Egri G, Palovics E, Kiss V (2006) Org Biomol Chem 4:3011–3030. doi:10.1039/b603058k
Jacques J, Collet A, Wilen SH (1994) Enantiomers, racemates and resolutions. Krieger Publishing Company, Malabar, pp 81, 296, 447 pp
Stahly GP (2007) Cryst Growth Des 7:1007–1026. doi:10.1021/cg060838j
Nangia A (2008) Cryst Growth Des 8:1079–1081. doi:10.1021/cg800198e
Suzuki TM, Ohba S, Sato S, Saito Y, Saito K (1983) Inorg Chem 22:2048–2054. doi:10.1021/ic00156a022
Jones P, Vagg RS, Williams PA (1984) Inorg Chem 23:4110–4111. doi:10.1021/ic00192a055
Mulqi MW, Williams PA, Stephens FS, Vagg RS (1984) Inorg Chim Acta 88:183–192. doi:10.1016/S0020-1693(00)83595-1
Fanwick PE, Kobriger LM, McMullen AK, Rothwell IP (1986) J Am Chem Soc 108:8095–8097. doi:10.1021/ja00285a040
Sheldrick WS, Heeb S (1990) Inorg Chim Acta 168:93–100. doi:10.1016/S0020-1693(00)88022-6
Alcock NW, Hulmes DI, Brown JM (1995) J Chem Soc Chem Commun 3:395–397. doi:10.1039/c39950000395
Bosch WH, Englert U, Pfister B, Stauber R, Salzer A (1996) J Organomet Chem 506:273–285. doi:10.1016/0022-328X(95)05690-Q
Ung VA, Bardwell DA, Jeffery JC, Maher JP, McCleverty JA, Ward MD et al (1996) Inorg Chem 35:5290–5299. doi:10.1021/ic951634n
Davies TJ, Carroll PJ, Walsh PJ (2002) J Organomet Chem 663:70–77. doi:10.1016/S0022-328X(02)01656-X
Mazet C, Gade LH (2003) Inorg Chem 42:210–215. doi:10.1021/ic025947b
Harding LP, Jeffery JC, Riis-Johannesen T, Rice CR, Zeng Z (2004) Dalton Trans 16:2396–2397. doi:10.1039/b407487b
Pelagetti P, Bacchi A, Calbiani F, Carcelli M, Elviri L, Pelizzi C et al (2005) J Organomet Chem 690:4602–4610. doi:10.1016/j.jorganchem.2005.07.028
He H, Lipowska M, Xu X, Taylor AT, Carlone M, Marzilli LG (2005) Inorg Chem 44:5437–5446. doi:10.1021/ic0501869
Dreos R, Mechi L, Nardin G, Randaccio L, Siega P (2005) J Organomet Chem 690:3815–3821. doi:10.1016/j.jorganchem.2005.05.017
Brunner H, Weber M (2003) Angew Chem Int Ed 42:1859–1862. doi:10.1002/anie.200250181
Lampe JW, Chou Y-L, Hanna RG, DiMeo SV, Erhardt PW, Hagedorn AA et al (1993) J Med Chem 36:1041–1047. doi:10.1021/jm00060a012
Yu Q, Baroni TE, Liable-Sands L, Rheingold AL, Borovik AS (1998) Tetrahedron Lett 39:6831–6834. doi:10.1016/S0040-4039(98)01486-5
Walker M, Pohl E, Herbst-Irmer R, Gerlitz M, Rohr J, Sheldrick GM (1999) Acta Crystallogr B B55:607–616. doi:10.1107/S0108768199003948
Kostyanovsky RG, Krutius ON, Bronzova IA, Lenev DA, Lyssenko KA, Averkiev BB (2001) Mendeleev Commun 1:6–8. doi:10.1070/MC2001v011n01ABEH001396
Saloutina LV, Zapevalov AY, Kodess MI, Lyssenko KA, Antipin MY, Saloutine VI et al (2003) J Fluor Chem 120:41–47. doi:10.1016/S0022-1139(02)00283-X
Linden A, Gündüz MG, Simsek R, Safak C (2006) Acta cryst. Sect C Crystallogr Struct Commun C62:o227–o230. doi:10.1107/S0108270106007530
Nonius BV (1998) COLLECT Program Package. Delft, The Netherlands
Otwinowski Z, Minor W (1997) Methods Enzymol 276:307–326. doi:10.1016/S0076-6879(97)76066-X
Otwinowski Z, Borek D, Majewski W, Minor W (2003) Acta Crystallogr A 59:228–234
Altomare A, Burla MC, Camalli M, Cascarano GL, Giacovazzo C, Guagliardi A et al (1999) J Appl Cryst 32:115–119. doi:10.1107/S0021889898007717
Farrugia JL (1999) J Appl Cryst 32:837. doi:10.1107/S0021889899006020
Sheldrick GM SHELX-97: Programs for crystal structure analysis, University of Göttingen, Institut für Anorganische Chemie der Universität, Tammanstrasse 4, D-3400 Göttingen, Germany, 1997
Flack HD (1983) Acta Crystallogr A 39:876–881. doi:10.1107/S0108767383001762
Acknowledgments
This study was supported by the Russian Foundation for Basic Research, grants 06-03-32508, 06-03-32085 and 07-03-00617. The authors would like to thank Dr. R. Fröhlich and Prof. Dr. G. Erker for their support and for providing X-ray facilities at the University of Münster (Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor Viktor Naumov (1932–2007).
Rights and permissions
About this article
Cite this article
Alfonsov, V.A., Bredikhin, A.A., Bredikhina, Z.A. et al. First examples of the cocrystallization of diastereomers of chiral phosphorus compounds. Struct Chem 19, 873–878 (2008). https://doi.org/10.1007/s11224-008-9353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-008-9353-4