Abstract
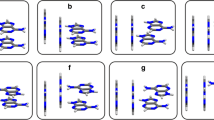
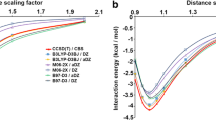
The stacking patterns of benzene and cytosine have been compared with the help of Bader’ theory of atoms in molecule using the conventional ab initio method. In addition, the differences in the stacking and hydrogen-bonded interactions in cytosine have also been quantified with the help of various topographical features of molecular electron density. The electron density at the bond critical point does not exhibit linear relationship with the strength of stacking interaction in various twisted cytosine dimers. However, the electron density and Laplacian of electron density at the cage critical point display good linear relationship with the stacking energy. The values of the electron density and Laplacian of electron density derived from AIM theory is highly useful in differentiating stacking and hydrogen-bonded interaction. The aromaticity of cytosine and cytosine stack has been compared with benzene and benzene stack employing NICS criteria.
Similar content being viewed by others
References
Muller-Dethlefs, K.; Hobza, P. Chem. Rev. 2000, 100, 143.
Karlstrom, G.; Linse, P.; Wallqvist, A.; Jonsson, B. J. Am. Chem. Soc. 1983, 105, 3777.
Carsky, P.; Selzle, H. L.; Schlag, E. W. Chem. Phys. 1988, 125, 165.
Hobza, P.; Selzle, H. L.; Schlag, E. W. J. Chem. Phys. 1990, 93, 5893.
Tsuzuki, S.; Uchimaru, T.; Tanabe, K. J. Mol. Struct. Theochem. 1994, 307, 107.
Hobza, P.; Selzle, H. L.; Schlag, E. W. J. Am. Chem. Soc. 1994, 116, 3500.
Jaffe, R. L.; Smith, G. D. J. Chem. Phys. 1996, 105, 2780.
Hobza, P.; Selzle, H. L.; Schlag, E. W. J. Phys. Chem. 1996, 100, 18790.
Spirko, V.; Engkvist, O.; Soldán, P.; Selzle, H. L.; Schlag, E. W.; Hobza, P. J. Chem. Phys. 1999, 111, 572.
Tsuzuki, S.; Uchimaru, T.; Sugawara, K.; Mikami, M. J. Chem. Phys. 2002, 117, 11216.
Henson, B. F.; Hartland, G. V.; Venturo, V. A.; Felker, P. M. J. Chem. Phys. 1992, 97, 2189.
Arunan, E.; Gutowsky, H. S. J. Chem. Phys. 1993, 98, 4294.
Sponer, J.; Hobza, P. Chem. Rev. 1999, 99, 3247.
Saenger, W. Principles of Nucleic Acid Structure; Springer-Verlag: New York, 1984.
Hunter, C. A. J. Mol. Biol. 1993, 230, 1025.
Jurecka, P.; Sponer, J.; Hobza, P. J. Phys. Chem. B. 2004, 108, 5466.
Sponer, J.; Hobza, P. Chem. Phys. Letts. 1997, 267, 263.
Sponer, J.; Gabb, H. A.; Leszczynski, J.; Hobza, P. Biophys. J. 1997, 73, 76.
Sponer, J.; Leszczynski, J.; Hobza, P. Biopolymers 2002, 61, 3.
Sponer, J.; Leszczynski, J.; Hobza, P. J. Phys. Chem. 1996, 100, 5590.
Sponer, J.; Leszczynski, J.; Hobza, P. J. Biomol. Struct. Dyn. 1996, 14, 117.
Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. J. Am. Chem. Soc. 2002, 124, 104.
Hobza, P.; Sponer, J.; Polasek, M. J. Am. Chem. Soc. 1995, 117, 792.
Amutha, R.; Subramanian, V.; Nair, B. U. Theor. Chem. Acc. 2002, 107, 343.
Sivanesan, D.; Babu, K.; Gadre, S. R.; Subramanian, V.; Ramasami, T. J. Phys. Chem. A. 2000, 104, 10887.
Popelier, P. L. A.; Joubert, L. J. Am. Chem. Soc. 2002, 124, 8725.
Parthasarathi, R.; Amutha, R.; Subramanian, V.; Nair, B.U.; Ramasami, T. J. Phys. Chem. A. 2004, 108, 3817.
Bader, R. F. W. Atoms in Molecules: A Quantum Theory; Oxford: Clarendon, 1990.
Bader, R. F. W. J. Phys. Chem. A. 1998, 102, 7314.
Bone, R. G. A.; Bader, R. F. W. J. Phys. Chem. 1996, 100, 10892.
Carroll, M. T.; Bader, R. F. W. Mol. Phys. 1988, 65, 695.
Bader, R. F. W.; Bayles, D. J. Phys. Chem. A. 2000, 104, 5579.
Popelier, P. L. A. Atoms in Molecules: An Introduction; Prentice Hall: New York, 2000.
Popelier, P. L. A. Coord. Chem. Rev. 2000, 197, 169.
Koch, U.; Popelier, P. L. A. J. Phys. Chem. 1995, 99, 9747.
Popelier, P. L. A. J. Phys. Chem. A. 1998, 102, 1873.
Popelier, P. L. A.; Joubert, L.; Kosov, D. S. J. Phys. Chem. A. 2001, 105, 8254.
Grabowski, S. J. Chem. Phys. Lett. 1999, 312, 542.
Grabowski, S. J. J. Phys. Chem. A. 2000, 104, 5551.
Grabowski, S. J. J. Phys. Chem. A. 2001, 105, 10739.
Grabowski, S. J. J. Mol. Struct. 2001, 562, 137.
Scheiner, S.; Grabowski, S. J.; Kar, T. J. Phys. Chem. A. 2001, 105, 10607.
Wojtulewski, S.; Grabowski, S. J. J. Mol. Struct. 2002, 605, 235.
Grabowski, S. J. J. Mol. Struct. 2002, 615, 239.
Wojtulewski, S.; Grabowski, S. J. J. Mol. Struct. 2003, 645, 287.
Wojtulewski, S.; Grabowski, S. J. J. Mol. Struct. Theochem. 2003, 621, 285.
Cubero. E.; Orozco, M.; Hobza, P.; Luque, F. J. J. Phys. Chem. A. 1999, 103, 6394.
Malcom, N. O. J.; Popelier, P. L. A. J. Phys. Chem. A. 2001, 105, 7638.
Hobza, P.; Sponer, J.; Cubero, E.; Orozco, M.; Luque, F. J. J. Phys. Chem. B. 2000, 104, 6286.
Munoz, J.; Sponer, J.; Hobza, P.; Orozco, M.; Luque, F. J. J. Phys. Chem. B. 2001, 105, 6051.
Sponer, J.; Leszczynski, J.; Hobza, P. J. Phys. Chem. 1996, 100, 1965.
Frisch, M. J. et al. Gaussian 98, Revision A.7; Gaussian, Inc.: Pittsburgh, PA, 1998.
Biegler-Konig, F.; Schonbohm, J.; Derdau, R.; Bayles, Bader, R. W. F. D. AIM 2000, version 1; Bielefeld: Germany, 2000.
Boys, S. F.; Bernardi, F. Mol. Phys. 1970, 19, 553.
Schleyer, P. v. R.; Maercker, C.; Dransfield, A.; Jiao, H.; Hommes, N. J. R. v. E. J. Am. Chem. Soc. 1996, 118, 6317.
Schleyer, P. v. R.; Jiao, H.; Hommes, N. J. R. v. E.; Malkin, V. G.; Malkina, O. L. J. Am. Chem. Soc. 1997, 119, 12669.
Schleyer, P. v. R.; Manoharan, M.; Wang, Z. X.; Kiran, B.; Jiao, H.; Puchta, R.; Hommes, N. J. R. v. E. Org. Lett. 2001, 3, 2465.
Koristsanszky, T. S.; Mallion, R. B. Chem. Rev. 2001, 101, 1583.
Howard, S. T.; Krygowski, T. M. Can. J. Chem. 1997, 75, 7813.
Ranganathan, A.; Kulkarni, G. U. J. Phys. Chem. A. 2002, 106, 7813.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parthasarathi, R., Subramanian, V. Stacking Interactions in Benzene and Cytosine Dimers: From Molecular Electron Density Perspective. Struct Chem 16, 243–255 (2005). https://doi.org/10.1007/s11224-005-4455-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11224-005-4455-8