Abstract
A clean, convenient and facile approach for one-pot ultrasonic assisted synthesis of substituted 3-pyrrolin-2-ones from diethyl acetylenedicarboxylate, aniline and aldehyde derivatives is described. The reactions were carried out in the presence of an efficient, green and reusable acidic nanocatalyst, magnetic cobalt ferrite nanoparticles functionalized with citric acid (CoFe2O4@CA), with high yields under mild conditions. Also, use of ultrasound irradiation made this approach an attractive protocol for the synthesis of these products. The method has been successful in achieving the green chemistry objective. An energy efficient protocol using ultrasound irradiation instead of conventional heating or stirring and use of ethanol as a non-hazardous, inexpensive and green solvent in the one-step reaction against sequential reaction steps thus combining the features of both economic and environmental advantages.
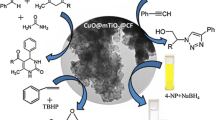
Graphic abstract
In this research, magnetic cobalt ferrite nanoparticles functionalized with citric acid (CoFe2O4@CA) catalyzed synthesis of substituted 3-pyrrolin-2-ones under ultrasonic irradiation via a practical and environmentally benign one-pot three-component protocol.










Similar content being viewed by others
References
R.C. Cioc, E. Ruijter, R.V. Orru, Green Chem. 16, 6 (2014)
H. Zhang, Catal. Lett. 144, 5 (2014)
L.F. Gutierrez, E. Nope, H.A. Rojas, J.A. Cubillos, A.G. Sathicq, G.P. Romanelli, J.J. Martínez, Res. Chem. Intermed. 44, 5559–5568 (2018)
C. Capello, U. Fischer, K. Hungerbühler, Green Chem. 9, 9 (2007)
H. Ahankar, A. Ramazani, K. Ślepokura, T. Lis, S.W. Joo, Green Chem. 18, 12 (2016)
D. Wang, D. Astruc, Chem. Rev. 114, 14 (2014)
M.B. Gawande, R. Luque, R. Zboril, ChemCatChem 6, 12 (2014)
H. Ahankar, A. Ramazani, S.W. Joo, Res. Chem. Intermed. 42, 3 (2016)
J.J. Martínez, E. Nope, H. Rojas, J. Cubillos, Á.G. Sathicq, G.P. Romanelli, Catal. Lett. 144, 7 (2014)
S. Zolfagharinia, E. Kolvari, N. Koukabi, Catal. Lett. 147, 6 (2017)
J.K. Rajput, G. Kaur, Catal. Sci. Technol. 4, 1 (2014)
K.K. Senapati, C. Borgohain, P. Phukan, J. Mol. Catal. A Chem. 339, 1 (2011)
A. Bazgir, G. Hosseini, R. Ghahremanzadeh, ACS Comb. Sci. 15, 10 (2013)
L.H. Abdel Rahman, A.M. Abu-Dief, R.M. El-Khatib, S.M. Abdel-Fatah, A. Adam, E. Ibrahim, Appl. Organomet. Chem. 32, 3 (2018)
F. Sadri, A. Ramazani, A. Massoudi, M. Khoobi, R. Tarasi, A. Shafiee, V. Azizkhani, L. Dolatyari, S.W. Joo, Green Chem. Lett. Rev. 7, 3 (2014)
M. Kooti, M. Afshari, Catal. Lett. 142, 3 (2012)
S.Y. Srinivasan, K.M. Paknikar, D. Bodas, V. Gajbhiye, Nanomedicine 13, 1221–1238 (2018)
G. Baldi, D. Bonacchi, M.C. Franchini, D. Gentili, G. Lorenzi, A. Ricci, C. Ravagli, Langmuir 23, 7 (2007)
R.A. Bohara, N.D. Thorat, H.M. Yadav, S.H. Pawar, N. J. Chem. 38, 7 (2014)
A. Meyers, L. Snyder, J. Org. Chem. 58, 1 (1993)
A.G. Malykh, M.R. Sadaie, Drugs 70, 3 (2010)
L.P. Dwoskin, L. Teng, S.T. Buxton, P.A. Crooks, J. Pharmacol. Exp. Ther. 288, 3 (1999)
P.N. Patsalos, Epilepsia 46, 140–148 (2005)
P. Singh, V. Dimitriou, R. Mahajan, A. Crossley, Br. J. Anaesth. 71, 5 (1993)
F. Haaf, A. Sanner, F. Straub, Polym. J. 17, 1 (1985)
R.H. Feling, G.O. Buchanan, T.J. Mincer, C.A. Kauffman, P.R. Jensen, W. Fenical, Angew. Chem. Int. Ed. 42, 3 (2003)
S. Omura, T. Fujimoto, K. Otoguro, K. Matsuzaki, R. Moriguchi, H. Tanaka, Y. Sasaki, J. Antibiot. 44, 1 (1991)
Y. Asami, H. Kakeya, R. Onose, A. Yoshida, H. Matsuzaki, H. Osada, Org. Lett. 4, 17 (2002)
A.L. Harreus, R. Backes, J.O. Eichler, R. Feuerhake, C. Jäkel, U. Mahn, R. Pinkos, R. Vogelsang, Ullmann’s Encycl. Ind. Chem. 1–7 (2011)
K. Ma, P. Wang, W. Fu, X. Wan, L. Zhou, Y. Chu, D. Ye, Bioorg. Med. Chem. Lett. 21, 22 (2011)
Y. Geng, X. Wang, L. Yang, H. Sun, Y. Wang, Y. Zhao, R. She, M.-X. Wang, D.-X. Wang, J. Tang, PLoS ONE 10, 6 (2015)
V. Koz’minykh, N. Igidov, S. Zykova, V. Kolla, N. Shuklina, T. Odegova, Pharm. Chem. J. 36, 4 (2002)
V. Gein, V. Mihalev, N. Kasimova, E. Voronina, M. Vakhrin, E. Babushkina, Pharm. Chem. J. 41, 4 (2007)
V. Gein, V. Yushkov, N. Kasimova, N. Shuklina, M.Y. Vasil’eva, M. Gubanova, Pharm. Chem. J. 39, 9 (2005)
V. Gein, M. Armisheva, N. Rassudikhina, M. Vakhrin, E. Voronina, Pharm. Chem. J. 45, 3 (2011)
CrysAlisCCD and CrysAlisRED in KM4-CCD software. Oxford Diffraction Ltd.: Yarnton, Oxfordshire, England (2010)
G.M. Sheldrick, Acta Crystallogr. Sect. A Found. Adv. 71, 1 (2015)
G.M. Sheldrick, Acta Crystallogr. Sect. C Struct. Chem. 71, 1 (2015)
K. Brandenburg, DIAMOND Version 3.2k. Crystal Impact GbR, Bonn, Germany (2014)
S.Y. Zhao, D.-G. Lee, C.-W. Kim, H.-G. Cha, Y.-H. Kim, Y.-S. Kang, Bull. Korean Chem. Soc. 27, 2 (2006)
J. Sun, Q. Wu, E.Y. Xia, C.G. Yan, Eur. J. Org. Chem. 2011, 16 (2011)
C.R. Groom, I.J. Bruno, M.P. Lightfoot, S.C. Ward, Acta Crystallogr. Sect. B Struct. Sci. 72, 2 (2016)
A. Ramazani, H. Ahankar, K. Ślepokura, T. Lis, S.W. Joo, J. Struct. Chem. 60, 662–670 (2018)
Acknowledgements
This work was supported by the “Iran National Science Foundation”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahankar, H., Ramazani, A., Ślepokura, K. et al. Magnetic cobalt ferrite nanoparticles functionalized with citric acid as a green nanocatalyst for one-pot three-component sonochemical synthesis of substituted 3-pyrrolin-2-ones. Res Chem Intermed 45, 5007–5025 (2019). https://doi.org/10.1007/s11164-019-03878-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03878-1