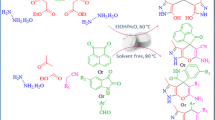
Abstract
We have studied the catalytic ability of copper(II) acetate monohydrate as a mild, environmentally benign, natural and economical catalyst for the multi-component efficient synthesis of biologically active spiro-4H-pyran derivatives and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives with excellent yields and short reaction times. The most important advantages of this procedure are its mild, non-toxic and inexpensive catalyst, one-pot synthesis, environmentally benign nature, solvent-free conditions, simple operational procedures, and highly efficient conditions.
Graphical Abstract






Similar content being viewed by others
References
M.M. Garima Kumari, Eur. J. Med. Chem. 46, 1181 (2011)
V.V. Vintonyak, K. Warburg, H. Kruse, S. Grimme, K. Hübel, D. Rauh, H. Waldmann, Angew. Chem. Int. Ed. Engl. 49, 5902 (2010)
J.S. Kim, H.K. Rhee, H.J. Park, S.K. Lee, C.O. Lee, H.Y. Park Choo, Bioorg. Med. Chem. 16, 4545 (2008)
S. Grasso, G. Desarro, N. Micale, M. Zappala, G. Puia, M. Baraldi, C. Demicheli, J. Med. Chem. 43, 2851 (2000)
M.M.-C. Lo, C.S. Newmann, S. Nagayams, E.O. Perlstein, S.L. Schreiber, J. Am. Chem. Soc. 127, 10130 (2005)
J. Li, Y.F. Zhau, X.Y. Yuan, J.X. Xu, I. Gong, Molecules 11, 574 (2006)
S. Yu, D. Qin, S. Shangary, J. Chen, G. Wang, K. Ding, D. McEachern, S. Qiu, Z. Nikolovska-Coleska, R. Miller, S. Kang, D. Yang, S. Wang, J. Med. Chem. 52, 7970 (2009)
A. Fensome, W.R. Adams, A.L. Adams, T.J. Berrodin, J. Cohen, C. Huselton, A. Illenberger, J.C. Kern, V.A. Hudak, M.A. Marella, E.G. Melenski, C.C. McComas, C.A. Mugford, O.D. Slayden, M. Yudt, Z. Zhang, P. Zhang, Y. Zhu, R.C. Winneker, J.E. Wrobel, J. Med. Chem. 51, 1861 (2008)
K. Rad-Moghadam, L. Youseftabar-Miri, Tetrahedron 67, 5693 (2011)
N. Azizi, S. Dezfooli, M.M. Hashemi, J. Mol. Liq. 194, 62 (2014)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763 (2014)
R. Sridhar, B. Srinivas, B. Madhav, V.P. Reddy, Y.V.D. Nageswar, K.R. Rao, Can. J. Chem. 87, 1704 (2009)
S.J. Chai, Y.F. Lai, J.C. Xu, H. Zheng, Q. Zhu, P.F. Zhang, Adv. Synth. Catal. 353, 371 (2011)
Y.B. Wagh, Y.A. Tayade, S.A. Padvi, B.S. Patil, N.B. Patil, D.S. Dalal, Chin. Chem. Lett. 26, 1273 (2015)
B. Maheshwar Rao, G. Niranjan Reddy, T. Vijaikumar Reddy, B.L.A. Prasad, R.B.N. Prabhavathi Devi, J.S. Yadav, B.V. Subba Reddy, Tetrahedron Lett. 54, 2466 (2013)
M. Saeedi, M.M. Heravi, Y.S. Beheshtiha, A. Oskooie, Tetrahedron 66, 5345 (2010)
D.S. Raghuvanshi, K.N. Singh, Tetrahedron Lett. 52, 5702 (2011)
M.R. Nabid, S.J.T. Rezaei, R. Ghahremanzadeh, A. Bazgir, Ultrason. Sonochem. 17, 159 (2010)
S.H. Song, J. Zhong, Y.H. He, Z. Guan, Tetrahedron Lett. 53, 7075 (2012)
R. Ghahremanzadeh, G. Imani Shakibaei, A. Bazgir, Synlett 8, 1129 (2008)
J. Safaei Ghomi, H. Shahbazi Alavi, A. Ziarati, R. Teymuri, M.R. Saberi, Chin. Chem. Lett. 25, 401 (2014)
Y.D. Reddy, B. Suryanarayana, C.V.R. Reddy, P.K. Dubey, Heterocycl. Lett. 4, 341 (2014)
G. Shanthi, G. Subbulakshmi, P.T. Perumal, Tetrahedron 63, 2057 (2007)
M. Veeranarayana Reddy, P. Chenna Rohini Kumar, G. Chandra Sekhar Reddy, C. Suresh Reddy, C. R. Chim. 17, 1250 (2014)
G. Mohammadi Ziarani, N. Hosseini Mohtasham, A. Badiei, N. Lashkari, J. Chin. Chem. Soc. (2014). doi:10.1002/jccs.201300538
M. Sayyafi, M. Seyyedhamze, H.R. Khavasi, A. Bazgir, Tetrahedron 64, 2375 (2008)
E. Mosaddegh, A. Hassankhani, Tetrahedron Lett. 52, 488 (2011)
L. Torkian, M. Dabiri, P. Salehi, M. Bararjanian, Helv. Chim. Acta 94, 1416 (2011)
Z. Liqin, Z. Bo, L. Yiqun, Chin. J. Org. Chem. 31, 553 (2011)
A. Strecker, Leibigs. Ann. Chem. 7, 27 (1850)
Z. Madanifar, M.T. Maghsoodlou, M. Kangani, N. Hazeri, Res. Chem. Intermed. 41, 9863 (2015)
P. Iniyavan, S. Sarveswari, V. Vijayakumar, Res. Chem. Intermed. 41, 7413 (2015)
F. Mohamad Pour, M.T. Maghsoodlou, R. Heydari, M. Lashkari, Iran. J. Catal. 6, 127 (2016)
N. Xiao, S.H. Wang, A.Y. Zhang, H.Y. Li, P. Wang, W. Li, B.H. Chen, G.F. Chen, N. Li, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-1961-1
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, Res. Chem. Intermed. 41, 2503 (2015)
S. Salahi, M.T. Maghsoodlou, N. Hazeri, F. Movahedifar, R. Doostmohammadi, M. Lashkari, Res. Chem. Intermed. 41, 6477 (2015)
Xu Xiaowen, Yiqun Li, Res. Chem. Intermed. 41, 4169 (2015)
M.T. Maghsoodlou, N. Khorshidi, M. Mousavi, N. Hazeri, S.M. Habibi-Khorassani, Res. Chem. Intermed. 41, 7497 (2015)
B. Mirhosseini-Eshkevari, M.A. Ghasemzadeh, J. Safaei-ghomi, Res. Chem. Intermed. (2015). doi:10.1007/s11164-014-1854-8
G. Mohammadi Ziarani, N. Hosseini Mohtasham, N. Lashgari, A. Badiei, Res. Chem. Intermed. 41, 7581 (2015)
L. Lv, S. Zheng, X. Cai, Z. Chen, Q. Zhu, S. Liu, J. Acs. Comb. Sci. 15, 183 (2013)
X. Xin, D. Xiang, J. Yang, Q. Zhang, F. Zhou, D. Dong, J. Org. Chem. 78, 11956 (2013)
W. Zhang, X. He, B. Ren, Y. Jiang, Z. Hu, Tetrahedron Lett. 56, 2472 (2015)
Acknowledgments
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamadpour, F., Maghsoodlou, M.T., Heydari, R. et al. Copper(II) acetate monohydrate: an efficient and eco-friendly catalyst for the one-pot multi-component synthesis of biologically active spiropyrans and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives under solvent-free conditions. Res Chem Intermed 42, 7841–7853 (2016). https://doi.org/10.1007/s11164-016-2565-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2565-0