Abstract
A one-dimensional model, LOGEcat is used to develop a detailed surface reaction mechanism for modeling the steam reforming of methane over a nickel-based catalyst. The focus of the paper is to develop a kinetically consistent surface reaction mechanism. The two terms, kinetically and thermodynamically consistent mechanisms, will be used frequently in this article. Note that when the mechanism is thermodynamically consistent then the thermodynamic data or the thermochemistry of the species is not used and all the reactions in the mechanism are forward reactions (no backward reactions included) which need the Arrhenius parameters (pre-exponential factor, \(A_{r}\); activation energy, \(E_{r}\); temperature exponent, \(\beta _r\)) explicitly for each reaction. The kinetically consistent mechanisms need Arrhenius parameters only for the forward reactions and does not need these parameters for backward reactions making the backward reactions independent of the kinetics. The rate for the backward reactions is calculated with the help of thermochemistry of the intermediate species involved in the reaction mechanism. Since the thermochemistry of the intermediate species are not easily available, this makes such studies much more complex. The original multi-step reaction mechanism consists of 42 reactions which are thermodynamically consistent. In this study we have modified this reaction mechanism from literature and used only 21 (reversible) direct reactions. This aspect is important because by using only 21 reactions, we use the thermochemistry of the species to achieve thermodynamic equilibrium instead of providing the Arrhenius parameters for forward and backward reactions. We use the same \(A_{r}\), \(E_{r}\) and \(\beta _r\) values as used in the reference paper for the considered 21 reactions which are in equilibrium. However, the value of \(A_{r}\), \(E_{r}\) and \(\beta _r\) for the inverse/backward reactions are not given in the mechanism and the reverse rates are calculated by using the equilibrium and forward rate. Therefore, a sensitivity analysis on thermochemistry of the species is performed for a better agreement with the literature data. The method is presented for ensuring kinetic consistency and bringing thermochemistry of species in play while developing a surface reaction mechanism. The applicability of the mechanism is tested for two different simulation set-ups available in literature considering various reactor conditions in terms of parameters given as temperature and steam-to-carbon (S/C) ratio. Several chemical reaction terms, such as, selectivity, conversion and \(\mathrm {H_2}/{\text{CO}}\) ratio are shown for different parameters in comparison with the available reference data. The detailed mechanism developed in this study is able to accurately express the steam reforming of methane over the nickel catalyst for complete ranges of temperature for both the set-ups and within acceptable limit for S/C ratio considered for the analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methane from natural gas is thought to be a clean interim fossil fuel on our way to \(\mathrm {CO_2}\) free energy supply. Due to its huge green house gas potential, methane needs to be captured wherever full combustion cannot be guaranteed. A promising technique to avoid methane slip is steam reforming of methane to produce syngas that can be used energetically or as a raw material.
The most common catalytic technologies for converting natural gas to synthesis gas in various compositions involves processes, such as, steam reforming (SR), partial oxidation (POX), autothermal reforming (ATR) and dry reforming (DR) [1]. In industry, the steam reforming of natural gas has been carried out since 1930 for the production of synthesis gas, such as, \(\mathrm {H_2}\) and CO [2]. Nowadays the reforming of methane is getting renewed attention due to an extensive interest in operating with increasing amount of \(\mathrm {CO_2}\) in the feed [3, 4]. Synthesis gas is an important intermediate in the chemical industry for manufacturing valuable basic chemicals and synthetic fuels, methanol synthesis, oxo synthesis, and Fischer-Tropsch synthesis [1, 5,6,7]. Hydrogen separated from the synthesis gas is largely used in the manufacturing of a variety of petroleum hydrogenation processes, ammonia, and for power generation [8,9,10].
Conventional steam reformers deliver relatively high concentrations of hydrogen at high fuel conversion [1, 2, 5] and the reaction is highly endothermic which requires a large efficient external energy supply [11]. Additionally, the efficiency of the process is severely affected by the catalyst deactivation due to carbon formation [5].
Nickel is the most prominent and state-of-the-art catalyst for the reforming of natural gas in the industry [12]. Even if noble metals under oxidation and reforming conditions have been found to be less prone to coke formation [13], Ni-based catalysts are preferred due to fast turnover rates, ease of availability and low costs, although the use is limited by their tendency towards coke formation [14,15,16].
Modeling of catalytic steam reforming of natural gas has predominantly been establish on global kinetic expressions [17, 18] and thermodynamic calculations [19]. The majority of studies found in literature discusses the kinetic models for steam- or \(\mathrm {CO_2}\)-reforming in the gas phase.
To reach a profound understanding of the catalytic reforming of methane over nickel, it is essential to have a better understanding of the elementary steps involved in the reaction mechanism at a molecular level. Several techniques have been used to study the reforming of methane and different reaction mechanisms and corresponding kinetic models have been proposed [20]. Despite all the experimental and theoretical investigations, the detailed path for the conversion of methane to syngas remains a controversial issue [6]. In [2, 21], a reaction mechanism is proposed for the steam reforming of methane accompanied by water-gas shift reactions on a Ni/Mg\(\mathrm {Al_2}\mathrm {O_4}\) catalyst. Wei et al. [22] postulated a catalytic sequence for reactions of \(\mathrm {CH_4}\) with \(\mathrm {CO_2}\) and \(\mathrm {H_2O}\) on Ni/MgO catalysts. A micro-kinetic model for steam reforming reactions over a Ni/Mg\(\mathrm {Al_2}\mathrm {O_4}\) catalyst was proposed in [23] by reactions for \(\mathrm {CO_2}\) reforming of methane and deactivation by carbon formation.
Even though, a variety of detailed multi-step surface reaction mechanisms have been published, for example, modeling partial and total oxidation of hydrocarbons over Pt [21, 24], Ni [20, 23, 25,26,27], and Rh [28,29,30], there is no kinetic model available in literature which provides kinetically consistent reaction mechanism. The available surface reaction mechanisms considers only thermodynamic consistency.
The aim of this work is to provide a detailed surface reaction mechanism which is kinetically consistent and uses reversible reactions for steam reforming of methane over Ni-based catalysts. The original mechanism taken from [25] consists of 42 irreversible reactions and forms the basis for the present investigation. The thermodynamic analysis is conducted on 13 site species by utilized two sets of thermodynamic data, one from DETCHEM [20, 25] (detchem.com/mechanisms) and another from RMG (Reaction Mechanism Generator) data base [31] (rmg.mit.edu). Further, the results produced with the developed surface reaction mechanism in this work are validated by comparing against a surface reaction mechanism from [25] and another mechanism from [20] which consist of 52 elementary step reactions.
We also make a note between the differences in the most important references which are related with this work, i.e., [20, 25, 32] in comparison with the present paper. [25] deals with the development of a detailed surface reaction mechanism which is thermodynamic consistent. This mechanism does not utilize the thermochemistry of the species and the Arrhenius parameters for all 42 reactions are given. The monolithic reactor is used for their investigations. In [20], the configuration of the reactor is changed from monolithic to fixed bed. However, the detailed surface reaction mechanism is expected to perform well for both the configurations. The mechanism developed in [20] is the extended mechanism from [25] by adding 10 more reactions and this mechanism is also thermodynamically consistent. Moreover, the mechanism from [20] can be used for investigations in several processes, such as, steam reforming, dry reforming, catalytic partial oxidation of methane unlike the mechanism developed in [25] which is only validated for steam reforming process. [32] presented a new one-dimensional modeling approach, LOGEcat and the findings of [25] are re-calculated using their published mechanism with the new model. The main idea behind [32] was to test the LOGEcat model performance so that we can use this model in future for complex investigations. Note that in [32], only the modeling approach is new and not the mechanism.
In this paper, we use the same modeling approach as in [32], however, the mechanism is taken from [25] and modified. Since the main target for the present study is to achieve a detailed surface reaction mechanism which is kinetically consistent, we do not vary the configuration of the reactor and use the monolithic reactor. In this study, the Arrhenius parameters for the forward reactions remains same as in [25], only the thermochemistry of the species are modified. The results obtained with this mechanism are validated against literature [20, 25]. The comparison of new results with [25] tests the predictability of the developed mechanism using a monolithic reactor in the considered temperature and fuel ratio range. Whereas the comparison of our results with [20] checks the performance of the mechanism even for a different reactor configuration (fixed bed) and in a wide range of temperature. The investigation is done only for the steam reforming of methane over a nickel catalyst.
Model Description
The 1D model, LOGEcat [33] is utilized to carry out the simulations. The model is based on the single-channel 1D catalyst model and is applicable to the simulation of all standard after-treatment catalytic processes of combustion exhaust gas. The model has been successfully used in past studies [34,35,36]. A detailed investigation of the steam reforming of methane over nickel in a generalised sense has been considered in [32]. Here we extend our earlier work from [32] to develop a detailed reaction mechanism which is kinetically consistent.
The mathematical treatment of the model is presented in our previous publication [32]. In this section, we summarize the important equations without considering the details. We have provided a list of abbreviations and symbols used in this section or elsewhere in [32]. We refer the reader to [32, 33] for more details.
Single Channel Model
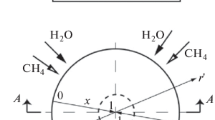
Fig. 1 shows the single channel which is divided into a finite number of cells with \(\Delta x\) as their length and each cell is treated as a perfectly stirred reactor (PSR). The pressure gradient along with inhomogeneity of the mixture can be neglected because the diameter of the catalytic channel is small. The external diffusion is modeled by a thin layer represented by a separate pore gas zone close to the wall.
The 1D model used to carry out the simulations is a part of the LOGEsoft software suite [33] for chemical reaction calculations. The conservation equations are given in the next section and these equations (gas species mass fraction, gas enthalpy, surface enthalpy, pore layer gas species mass fraction, and surface site fractions) are solved in each PSR and for each time step. Additionally, the 1D Navier-Stokes equations for flow velocity are solved by an operator splitting method over all cells.
Conservation Equations
In the operator splitting loop, the bulk gas in each cell is modeled by a PSR with the constant pressure assumption during the time step \(\Delta t\). The species transport between bulk gas as well as pore volume layer is accounted by the mass transfer coefficient and the conservation equation for bulk gas species mass fraction is given as,
The pore volume layer considers gas phase as well as surface reactions which further includes diffusion into the pores and the conservation equation of the gas phase species is given as,
The conservation equation for surface species site fraction is given as,
The bulk energy (specific enthalpy) takes in to account the heat transport by convection and molecular transport. The conservation equation is given as,
The pressure is assumed constant in the pore layer. The pore layer temperature is assumed to be homogeneous for the substrate as well as for the gas. The kinetic energy due to gas movement is neglected. With the stated assumptions, the conservation equation for the surface enthalpy is given as,
The mass (\(k_{m,i}\)) and heat (\(h_T\)) transfer coefficients used in the conservation equations are calculated from the Sherwood and Nusselt numbers [37] and for simultaneously developing velocity, concentration and thermal boundary layer flow, the correlations for Sherwood and Nusselt numbers are used as [38],
The Schmidt number for species i, \(Sc_i\) and the Prandtl number, Pr are given as,
Flow Equations
Assuming that the flow is in steady state, the conservation equations are given as,
the mass and momentum equations, respectively. The friction factor, \(f_F\) for laminar and fully developed flow is given below,
For more details related to the model and derivations for the equations, we refer the reader to [33].
Surface Reaction Mechanism
The conversion of methane and steam into a mixture of hydrogen, carbon monoxide and carbon dioxide can be examined as a combination of several reactions. This section covers the detailed surface reaction mechanism which is kinetically consistent.
The starting mechanism is adopted from Maier et al. [25] to model reforming of methane on nickel which contains 6 gas phase species and 13 surface species. The thermodynamic data for all the surface species from DETCHEM and RMG is summarised in Table 1. The initial reaction mechanism consists of 42 elementary reactions [25] resulting in a detailed reaction mechanism containing 21 reversible reactions developed in the present study (see Table 2).
The reaction mechanism suggests that adsorbed carbon species (\(\mathrm {C}\), \(\mathrm {CH}\), \(\mathrm {CH_2}\), \(\mathrm {CH_3}\)) formed from activated methane react with adsorbed atomic oxygen O(s), formed from the adsorption of oxygen or from the decomposition of water and \(\mathrm {CO_2}\), and produce carbon oxide.
The surface reaction mechanism initially consisting of overall 42 explicit forward and backward reactions is reduced to 21 reversible reactions by making it equilibrium. The calculation of the reverse reaction rates is explained with the help of equations discussed below. The calculation for the reverse reaction rates depends on the forward reaction rate and thermochemistry of the species. We use the term kinetically consistent mechanism for the proposed mechanism. Here, we have utilized two sets of thermodynamic data, one from DETCHEM [20, 25] and another from RMG data base [31].
In first step, simulations were conducted using the thermodynamic data sets (DETCHEM and RMG) and results were compared against the reference data. It was found that, using both DETCHEM and RMG thermodynamic data set could not reproduce the conversion rate faithfully. In second step, we used DETCHEM thermodynamic data and replaced each site species sequentially from RMG. This narrowed down our search for the species which are most sensitive. We found that the species CO(s) and \(\mathrm {CH_4}\)(s) are the most sensitive in model predictions. From the identified species CO(s) was found to exhibit the highest sensitivity out of both. Further details are given in Sect. “Thermochemistry Sensitivity Analysis”. Due to CO(s) high sensitivity in model predictions we selected this species for model improvement. The enthalpy of formation of CO(s) is increased by 40 kJ and for the range of investigated conditions we found a good agreement against the experimental data.
Next, we summarise all the equations for calculating rate constant and the equilibrium of a chemical reaction is given as,
which is determined by the thermodynamic properties of the participating species i. In the present study, the homogeneous gas-phase reactions can be neglected and the chemical source terms due to catalytic reactions are modeled by elementary-step based reaction mechanisms [39]. Generally, it is assumed that the forward rate constant, \(k_{f,r}\), for the reactions r have the following Arrhenius temperature dependence, for gas-phase as well as surface species,
The thermodynamic data associated with each species in a reaction are used to calculate the equilibrium constant and the calculated equilibrium constant in turn is used for calculating the reverse rate coefficients for a reaction. The reverse rate constants, \(k_{b,r}\), in the thermal systems, are related to the forward rate constants through the equilibrium constants given below,
The equilibrium constant, \(K_{c,r}\), given in concentration units considers the surface state and the gas state in case of reactions involving surface species as,
The equilibrium constants, \(K_{p,r}\), in pressure units is given as,
S and H are calculated using thermodynamic data as,
To summarize, the rate constants in case of an irreversible reaction are calculated using Eq. (14). This means that the rate coefficients, \(A_{r}\), \(\beta _r\) and \(E_{r}\), are specified for all the reactions in the input file. Whereas, in case of a reversible reaction, Eq. (14) is used to calculate forward rate constant and Eq. (15) for reverse rate constant which also uses the equilibrium rate constant calculated from thermodynamic data.
Simulation Set-up
To validate and compare our model performance, we have utilized the two different experimental data set from Maier et al. [25] and Delgado et al. [20]. The geometric data and catalyst parameters used to perform the simulations are summarized in Tables 3 and 4 respectively.
For the set-up from Maier et al. [25], the simulations have been performed for different reactor conditions in terms of parameters, such as, temperature and S/C ratio. The summary of the simulation cases performed for set-up from [25] are given in Table 3. Analysis is done for four different temperatures, T = 920, 1020, 1120 and 1220 K while keeping S/C ratio fixed as 2.77 and flow rate (\({\dot{\mathrm{f}}}\)) as 593 mL/min. Additionally, the S/C ratio is varied as S/C = 1.9, 2.77 and 3.67, with temperature T = 1020 K as constant.
In another set-up from [20], the investigation is extended for temperature range T\(\epsilon\)400–1200 K in order to measure concentration for different species, for instance, \(\mathrm {CH_4}\), \(\mathrm {{H}_2O}\), \(\mathrm {H_2}\), \(\mathrm {CO}\) and \(\mathrm {{CO}_2}\). The simulations performed with this set-up for various cases are summarised in Table 4.
A single channel is considered which is uniformly divided into 25 cells and one layer of washcoat is used for the simulations. The overall heat transfer efficiency factor, mass transfer efficiency factor and efficiency factors for surface chemistry are taken as one. The surface site density, \(\tau\) for Ni is \(2.6\times 10^{-5}\) mol/\(\mathrm{{m}}^{2}\) for both the set-ups. The active catalytic surface area per catalyst length is adjusted by carrying out the sensitivity analysis. \(75\%\) Argon dilution is used in case of first set-up and for the second we have used Nitrogen dilution as \(96\%\). The geometric data and catalyst parameters used to carry out the simulations are provided in Table 5.
Simulation Results from First Set-Up
Some of the important terms encountered in chemical reaction engineering, such as, conversion defined as the ratio of how much of a reactant has reacted and selectivity as the ratio of the desired product to the undesired products are discussed in this section. The variation of these quantities are shown for both set-ups (shown in Tables 3 and 4) with different parameters in order to re-capture the basic chemistry and to check the predictability of the developed surface reaction mechanism. This will allow us to use the thermodynamic data. Hence, a sensitivity analysis on thermochemistry of the intermediate species was performed before performing the final simulation which is given in Sect. “Thermochemistry Sensitivity Analysis”.
Variation of Temperature
In this section, the simulation results are presented for 4 different temperatures at T = 920, 1020, 1120 and 1220 K. All the other physical and numerical parameters used to perform the simulations are given in the previous section (see Table 3, case M1–M4).
Fig. 2 displays model predictions for the conversion of methane and water as a function of temperature in comparison with the reference simulation and experimental data from Maier et al. [25] along with the equilibrium calculations for the reference data. The simulation results produced with the developed reaction mechanism are within the acceptable limit as compared to the experimental data. However, the \(\mathrm {H_2}\)O conversion with our model is over-predicted similar to the Maier et al. [25] model in comparison to the experiment at temperatures higher than 1100 K.
In Fig. 3, the \(\mathrm {CO}\) selectivity and \(\mathrm {H_2}\)/CO ratio in methane steam reforming is shown with the reference data with temperature variation. It can be observed that for CO selectivity the model predictions at low and high temperatures matches with the experimental data whereas at temperatures 1000 and 1100 K the model slightly over-predicts the profile as compared to the experimental data. However, opposite was noted in our previous investigation [32], where the model results at 1000 and 1100 K were matching with the experimental data showing deviations (under-predicted) at low as well as high temperatures. Nevertheless, the model follows the experimental trend qualitatively.
The \(\mathrm {H_2/CO}\) ratio shown in Fig. 3 using the 1D model lies on top of the equilibrium calculations from [25] with the kinetically consistent surface reaction mechanism whereas, in our previous study [32] this ratio was away from reference equilibrium. This might be because of the establishment of the equilibrium with the usage of the thermochemistry of the participating species in the developed mechanism. However, in [32], the equilibrium was achieved only with the Arrhenius parameters without using the thermodynamic data of the species. The \(\mathrm {H_2/CO}\) ratio for Maier et al. (2D simulations) and experimental results are over-predicted compared to the equilibrium calculation at all temperatures. The over-prediction of the considered profile at the given S/C ratio (2.77) might be due to the underestimation of water-gas shift reaction at low temperature [25]. Nonetheless, the qualitative behaviour for \(\mathrm {H_2/CO}\) ratio for 1D, 2D and experimental data is similar.
The methane conversion for fixed S/C ratio (S/C = 3), pressure and flow rate with temperature variation in wide range along with the reference data from [25] is shown in Fig. 4. The model result lies on top of the reference simulation and experimental data from [25] for temperatures lower than 800 K as well as higher than 1100 K. Whereas, in the middle temperature range (800–1040 K), the results produced with the kinetically consistent surface reaction mechanism are slightly under-predicted in comparison to the reference data. Note that the methane conversion with the thermodynamically consistent surface reaction mechanism reported in [32] showed same behaviour as with the kinetically consistent mechanism in the considered temperature range.
Variation of S/C Ratio
The variation of some of the above discussed quantities with changing S/C ratio is shown next to confirm if the developed reaction mechanism also holds for different reactor condition. The simulations are performed for three different S/C ratio as S/C = 1.9, 2.77 and 3.67 while keeping all the other parameters fixed as T = 1020 K, P = 1 atm, \({\dot{\mathrm{f}}}\) = 593 mL/min and \(75\%\) Argon dilution (see Table 3, case M5-M7). The reference data from 2D simulations and experimental measurements are also plotted.
In Fig. 5, the methane and water conversion as a function of S/C (inlet steam-to-carbon) ratio is displayed along with the reference data. The conversion profiles for both the reactants produced with the kinetically consistent surface reaction mechanism are more or less same as the previous finding reported in [32] where a thermodynamically consistent surface reaction mechanism was used. However, both approaches, kinetically as well as thermodynamically consistent, using LOGEcat gives acceptable conversion of water and over-predicts methane conversion as compared to the reference results. Nevertheless, the error is less than \(10\%\). We note that the developed reaction mechanism is capable to capture the chemistry correctly at different reactor conditions. The qualitative behaviour remains similar to the experiments, such that the methane conversion increases and the water conversion decreases with increasing S/C ratio. The methane conversion is approximately 80\(\%\) for low S/C ratio and this increases to \(\approx 97\%\) for S/C = 3.77 at the considered conditions. The methane conversion at medium temperatures was observed in the range of 55–75\(\%\) which is inline with the study of Maier et al. [25]. The water conversion, however, reduces from \(\approx 60\%\) at S/C = 2 to \(\approx 40\%\) at high S/C ratio.
The \(\mathrm {CO}\) selectivity and \(\mathrm {H_2}\)/CO ratio in methane steam reforming is depicted in Fig. 6 as a function of the S/C ratio for T = 1020 K along with the results from [25]. The thermodynamic equilibrium in the reaction needs to be considered to analyze the composition of the products. When the inlet steam-to-carbon ratio is less than the \(\mathrm {CO}\) selectivity is higher and it decreases with increasing S/C ratio. This varies in the range \(\approx 42{\text{-}}37\%\) for S/C = 2.0–3.77, respectively. The LOGEcat model predicts the \(\mathrm {CO}\) selectivity well for lower S/C ratio whereas, for higher fuel ratio, the selectivity is over-predicted as compared to the reference data by utilizing the developed reaction mechanism which is kinetically consistent. As reported in [32], an opposite trend for \(\mathrm {CO}\) selectivity was observed while using a thermodynamically consistent surface reaction mechanism. In [32], the CO selectivity agrees with reference experiments for high fuel ratio.
\(\mathrm {H_2}/CO\) ratio variation with fuel ratio is shown in Fig. 6. This profile is highly under-predicted for our simulations produced with the kinetically consistent surface reaction mechanism as compared to the reference data. This was expected because from Fig. 3, we note that the \(\mathrm {H_2}/{\text{CO}}\) with temperature variation agrees with the equilibrium calculations and hence something similar is anticipated for the fuel ratio variation. This hints that the developed mechanism preforms better when the reforming is at thermodynamic equilibrium. A further investigation is needed if the reforming is away from the equilibrium. Nevertheless, one needs reference equilibrium data for this profile to make better comparison but for the considered set-up we do not have any equilibrium reference data.
When the S/C ratio is varied for the considered mechanism, the 1D results show deviation with comparison to the 2D and experimental results from [25]. The agreement is within ±15\(\%\) error, not as good as with temperature variation. Note that the equilibrium results for S/C variation are not shown for the reference data. The developed mechanism needs further investigations to capture the reforming for the given conditions.
The analysis hints to investigate the mechanism further. We need to perform the sensitivity analysis on the thermochemistry of the species involved in the reaction mechanism in more detail to find the most sensitive species for various reactor conditions. We also recommend to look in the reaction pathways for the new mechanism to understand the reasons behind the deviations in model predictions as compared to the literature experiments.
Simulation Results from Second Set-Up
In this section, the concentrations of different species are discussed with the variation of temperature for a simulation set-up taken from [20] (see Table 4, case D1–D9). The simulations are performed for T\(\epsilon\)400–1200 K for 1 bar total pressure and flow rate at 4 slpm (T = 298.15 K and P = 1.01325 bar). The inlet gas mixture is used as \(\mathrm {CH_4}\) = 1.60, \(\mathrm {{H}_2O}\) = 2.00 and \(\mathrm {N_2}\) = 96.40 vol\(\%\) (mole based) for a fixed S/C ratio to 1.25.
In Fig. 7, the methane and water concentration computed with the 1D model as a function of temperature is shown. The reference data from [20] for the given species is also plotted. The simulated concentration of methane decreases with increasing temperature similar to the experimental trend. Methane is fully consumed at temperature \(\approx\) 900 K. It can be observed that the kinetically consistent surface reaction mechanism developed in this work produce concentration of methane in good agreement with the experimental data as well as the simulation results from Delgado et al. [20] quantitatively as well as qualitatively. It is worth noting that the Delgado et al. [20] used reaction mechanism consisting of 52 reactions (explicit forward and backward reactions), however our model consist of 21 reversible reactions which are kinetically consistent.
Methane and water concentration as a function of temperature with S/C = 1.25 along with the reference data from [20]
The water concentration profile predicted with the proposed mechanism (Fig. 7) shows similar behaviour as shown by the other reactant, methane. The profile shows similar behaviour as reference data from [20] qualitatively and within acceptable limit quantitatively. The water concentration reduces with the increasing temperature. The simulation results computed in the present study as well as from reference data, deviates from the experimental results. This is known that it is difficult to measure water in experiments accurately. Since simulation results from Delgado et al. [20] and our simulations are similar, this may hint towards the higher measurement uncertainty in experiment.
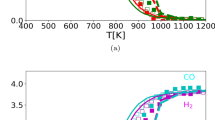
The concentration of the products is discussed next. Fig. 8 illustrates the \(\mathrm {H_2}\), CO and \(\mathrm {CO_2}\) concentration for the present investigation as a function of temperature along with the reference data from [20]. The concentration for hydrogen shows similar behaviour as shown by the reference data and is seen zero at low temperature, for T < 700 K. After T > 700 K, the formation of hydrogen starts which lasts up to T\(\approx\) 1000 K and then saturates at higher temperatures. The hydrogen concentration produced with the kinetically consistent surface reaction mechanism developed in the present work is in agreement with the reference data from [20].
\(\mathrm {H_2}\), CO and \(\mathrm {CO_2}\) concentration as a function of temperature with S/C = 1.25 along with the reference data from [20]
The CO concentration is also displayed in Fig. 8 as a function of temperature along with the reference data from [20]. The concentration for CO shows qualitatively similar behaviour as shown by the reference data. However, the concentration for CO using the new detailed surface reaction mechanism is slightly under-predicted in comparison with the reference experiments. Note that the reference simulations are also under-predicted as compared to the experiments and equilibrium calculations. In [20], it is concluded that the simplification of the used one-dimensional packed-bed model assuming isothermal conditions over the bed cross section introduces an error in the simulated species concentrations in comparison with the measured concentrations. However, it is worth noting that we have not used packed-bed to perform the simulations, instead we have considered a monolithic reactor configuration under steam reforming conditions. The modeling results presented in this paper may be different due to different simulating boundary conditions because we do not have isothermal conditions in LOGEcat. The focus of the present study is to develop a detailed reaction mechanism which is kinetically consistent and we do not aim to carry out simulations with packed-bed. We speculate that the difference in species concentration could be improved by considering a packed-bed configuration.
The concentration for \(\mathrm {CO_2}\) (Fig. 8) is also qualitatively similar in behaviour with the reference data. The concentration for this species is initially very low at low temperatures, then rises from T\(\epsilon\) 700–900 K and finally drops for T > 900 K for present simulations. The peak value for the \(\mathrm {CO_2}\) concentration in the present study is shifted at higher temperature (which is at T\(\approx\) 900 K) in comparison with the reference simulations and experimental data (capturing the \(\mathrm {CO_2}\) concentration peak at T\(\approx\) 750 K for experiments and T\(\approx\)800 K for reference simulations). Note that the shift of the peak towards higher temperature is also observed for reference simulations as compared to the experiments and equilibrium calculations shown in the figure. The overall concentration for \(\mathrm {CO_2}\) using the new reaction mechanism is under-predicted in comparison with the reference results. We speculate that the error in species concentrations might be due to the simplifications used in the one-dimensional model or it could be due to the simulation set-up used to perform the simulations. Nevertheless, the developed reaction mechanism consisting of only 21 reversible reactions is capable to capture the species concentration to a great extent for wide range of investigated conditions which makes the new mechanism robust, reliable and kinetically accurate.
Thermochemistry Sensitivity Analysis
In this section, the simulation results for thermochemistry sensitivity analysis for different species is discussed. Thermodynamic data from DETCHEM and RMG are used to perform the sensitivity analysis. The simulations are performed for 4 different temperatures at T = 920, 1020, 1120 and 1220 K and all the other physical and numerical parameters used to perform the simulations are kept fixed as given in the previous sections.
The conversion of methane and water as a function of temperature is shown in Fig. 9. The dashed line illustrates the 1D simulation results produced by using thermodynamic data from RMG and the dotted line from DETCHEM. For comparison, the reference data [25] for the cases considered is also plotted.
Methane and water conversion as a function of temperature while keeping all the other parameters fixed as S/C = 2.77, P = 1 atm, \({\dot{\mathrm{f}}}\) = 593 mL/min and \(75\%\) Argon dilution along with the reference data. The dashed line shows 1D simulation results produced by using thermodynamic data from RMG and the dotted line from DETCHEM
It can be observed in Fig. 9 using the complete thermochemistry set from DETCHEM and RMG that the model cannot predict the \(\mathrm {CH_4}\) and \(\mathrm {H_2}\)O conversion below 1100 K, while the model predictions using DETCHEM data are closer to experiments compared to RMG thermochemistry. Furthermore, above 1100 K, the model predictions are closer to experimental data using both thermochemistry sets. To investigate the thermochemistry sensitivity of each species, the DETCHEM thermochemistry set has been adopted as starting point for all 13 site species. The thermodynamic data of each species have been sequentially replaced with the thermochemistry data from RMG data. The results of the thermochemistry analysis are provided in an additional document.
Methane and water conversion as a function of temperature while keeping all the other parameters fixed as S/C = 2.77, P = 1 atm, \({\dot{\mathrm{f}}}\) = 593 mL/min and \(75\%\) Argon dilution along with the reference data. The dashed line shows 1D simulation results produced with raising the enthalpy of CO(s) by 10 kJ. Similarly, the dashed-dotted line depicts the 1D simulation results for raising the enthalpy of CO(s) by 40 kJ and the dotted line raising the enthalpy of the same species by 50 kJ
From the thermochemistry sensitivity of each species, it is observed that while using the thermodynamic data of species CO(s) and \(\mathrm {CH_4(s)}\) from RMG, model predictions are in closer agreement with the experimental data as compared to other species. For instance, model predictions are not in good agreement by replacing O(s), H(s), C(s) and \(\mathrm {CO_2(s)}\) thermochemistry (see additional document for sensitivity of these species). Further investigations are performed with the thermodynamic data from DETCHEM for all species except for CO(s) which is replaced with RMG data. It was found that adjusting CO(s) thermochemistry provided better model predictions against experimental data for the considered conversion profile.
Next, the enthalpy of formation sensitivity analysis is performed for species CO(s) whose thermodata are adopted from RMG. The simulations were performed by increasing the enthalpy of formation of CO(s) from 1 to 100 kJ. However, here we only show three cases. Fig. 10 shows the methane and water conversion as a function of temperature while keeping all the other parameters fixed as S/C = 2.77, P = 1 atm, \({\dot{\mathrm{f}}}\) = 593 mL/min and \(75\%\) Argon dilution along with the reference data. The dashed line in the figure shows the simulation results by increasing the enthalpy of CO(s) by 10 kJ, the dashed-dotted line by 40 kJ and the dotted line by 50 kJ. It can be observed that the model predictions are in much better agreement when enthalpy of CO(s) is increased by 40 kJ.
From the above analysis the thermochemistry of species CO(s) has been taken from RMG and adopted for our model development by increasing the enthalpy of formation by 40 kJ which overall provides a better agreement for the range of investigated conditions. Therefore, all the simulation results discussed in previous sections have been performed using changed thermochemistry of CO(s). (We have also provided an additional document which illustrates the comparison between the 1D simulation results with initial surface reaction mechanism from [25] consisting of 42 reactions and the developed surface reaction mechanism containing 21 reversible reactions.)
Conclusions
To summarize, we have used the calculation program as presented in [32] and modified the surface reaction mechanism from [25] to predict steam reforming of methane over nickel catalyst. Originally, the mechanism in [25] consists of 42 irreversible reactions with the Arrhenius parameters (\(A_{r}\), \(E_{r}\) and \(\beta _r\)) specified for each reaction individually and the equilibrium can be achieved by modifying these parameters. However, the optimum method to achieve thermodynamic equilibrium would be to use the reliable thermochemical data of the species because Arrhenius parameters are more accurate to define the speed of the reactions instead of establishing the equilibrium.
Since the thermochemistry of the gas phase species are easily available, it is very common to use all the reactions in equilibrium and thermochemistry of the species is used to calculate the reverse rates in gas phase reactions. However, in case of heterogeneous multi-step reaction mechanisms, the thermodynamic data for individual surface species are usually not available in literature and this problem leads to the development of several thermodynamically consistent mechanisms where one needs to specify Arrhenius parameters for forward as well as backward reactions and these parameters are modified to achieve equilibrium or to match experiments. While doing so, the thermochemistry of the intermediate species in not needed.
Whereas, in this paper our focus is not to adjust Arrhenius parameters but to modify thermochemistry of the species and make all the reactions in equilibrium. Hence, the reaction mechanism in [25] becomes our base and we used only the forward reactions from [25] with their Arrhenius parameters. All these reactions are made in equilibrium in order to use the thermochemistry of the species involved in the mechanism. We have used the thermochemistry of the species from two different sources [25, 31] and carried out the sensitivity analysis on Enthalpy of formation of the species to modify the thermodynamic data in order to develop a detailed surface reaction mechanism which is capable to produce the results with 21 reversible reactions instead of using 42 or 52 multi-step reactions. While adjusting the thermodynamic data, utmost care is taken to maintain the general reaction features of the new reaction mechanism. We have used a one-dimensional tool, LOGEcat, to develop this mechanism. In our previous work, we used all 42 reactions from [25] and tested the performance of our model, LOGEcat by comparing our results with the literature.
This work presents a detailed sensitivity analysis on thermochemistry of the species which helped us to develop a new kinetically consistent surface reaction mechanism. The study provides an updated thermodynamic data of the intermediate species which can be used in steam reforming or similar processes in future and this will also reduce the work of adjusting the Arrhenius parameters to achieve thermodynamic equilibrium.
We recommend further investigations to improve the results. This can be done by performing a more rigorous analysis on Enthalpy of formation of the species involved in the reaction mechanism by using thermodynamic data from other sources available in literature or also on Entropy which in turn will influence the Gibb’s free energy. Even the sensitivity limit of the Enthalpy of formation of species can be predicted. More reaction pathways can be added to make the mechanism applicable to other processes such as dry reforming and partial oxidation of methane over a nickel catalyst.
The results obtained with the new surface reaction mechanism are validated against two reactor configurations to check the performance of the mechanism in a wide range of temperature and fuel ratio for the steam reforming of methane over nickel catalyst. The simulated conversion, selectivity and \(\mathrm {H_2}/{\text{CO}}\) ratio at strongly varying conditions are well-computed for both the simulation set-ups considered with the 1D model using the developed detailed surface reaction mechanism.
Change history
30 October 2022
ESM file ‘sm_KineticConsist_chemkin.txt’ not included in the original publication
References
Rostrup-Nielsen JR, Sehested J, Nørskov JK (2002) Hydrogen and synthesis gas by steam- and \({\text{CO}}_2\) reforming. Adv Catal 47:65–139
Xu J, Froment GF (1989) Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. Am Inst Chem Eng AIChE 35:88–96. https://doi.org/10.1002/aic.690350109
Rostrup-Nielsen JR (1984). In: Anderson JR, Boudart M (eds) Catalytic steam reforming in catalysis-science and technology. Springer, Berlin
Rostrup-Nielsen JR, Hansen JHB (1993) CO2-reforming of methane over transition metals. J Catal 144:38–49. https://doi.org/10.1006/jcat.1993.1312
Rostrup-Nielsen JR (2000) New aspects of syngas production and use. Catal Today 63:159–164
Iglesia E (1997) Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl Catal A 161:59–78
Hickman DA, Schmidt LD (1993) Production of syngas by direct catalytic oxidation of methane. Science 259:343–346
Shao Z, Haile SM (2004) A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431:170–173
Park S, Vohs JM, Gorte RJ (2000) Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 404:265–267
Singhal SC (2000) Advances in solid oxide fuel cell technology. Solid State Ion 135:305–313
Michael BC, Donazzi A, Schmidt LD (2009) Effects of H2O and CO2 addition in catalytic partial oxidation of methane on Rh. J Catal 265:117–129. https://doi.org/10.1016/J.Jcat.2009.04.015
Schädel BT, Duisberg M, Deutschmann O (2009) Steam reforming of methane, ethane, propane, butane, and natural gas over a rhodium-based catalyst. Catal Today 142:42–51. https://doi.org/10.1016/j.cattod.2009.01.008
Ross JRH, vanKeulen ANJ, Hegarty MES et al (1996) The catalytic conversion of natural gas to useful products. Catal Today 30:193–199
Guo J, Lou H, Zheng XM (2007) The deposition of coke from methane on a NiMgAl2O4 catalyst. Carbon 45:1314–1321
Ginsburg JM, Pina J, El Solh T et al (2005) Coke formation over a nickel catalyst under methane dry reforming conditions: thermodynamic and kinetic models. Ind Eng Chem Res 44:4846–4854
Chen D, Lodeng R, Anundskas A et al (2001) Deactivation during carbon dioxide reforming of methane over ni catalyst: microkinetic analysis. Chem Eng Sci 56:1371–1379
Mbarawa M, Milton BE, Casey RT (2001) Experiments and modelling of natural gas combustion ignited by a pilot diesel fuel spray. Int J Therm Sci 40:927–936. https://doi.org/10.1016/S1290-0729(01)01279-0
Flowers D, Aceves S, Westbrook CK et al (2001) Detailed chemical kinetic simulation of natural gas HCCI combustion: gas composition effects and investigation of control strategies. J Eng Gas Turbins Power 123:433–439. https://doi.org/10.1115/1.1364521
Echigo M, Tabata T (2004) Simulation of the natural gas steam reforming process for PEFC systems. J Chem Eng Jpn 37:723–730. https://doi.org/10.1252/jcej.37.723
Delgado KH, Maier L, Tischer S et al (2015) Surface reaction kinetics of steam- and \({\text{CO}}_2\)-reforming as well as oxidation of methane over nickel-based catalysts. Catalysts 5:871–904. https://doi.org/10.3390/catal5020871
Quiceno R, Deutschmann O, Warnatz J et al (2006) Modelling of the high-temperature catalytic partial oxidation of methane over platinum gauze. Detailed gas-phase and surface chemistries coupled with 3D flow field simulations. Appl Catal A 303:166–176. https://doi.org/10.1016/j.apcata.2006.01.041
Wei J, Iglesia E (2004) Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J Catal 224:370–383. https://doi.org/10.1016/j.jcat.2004.02.032
Aparicio LM (1997) Transient isotopic studies and microkinetic modeling of methane reforming over nickel catalysts. J Catal 165:262–274. https://doi.org/10.1006/jcat.1997.1468
Chatterjee D, Deutschmann O, Warnatz J (2001) Detailed surface reaction mechanism in a three-way catalyst. Faraday Discuss 119:371–384. https://doi.org/10.1039/b101968f
Maier L, Schädel B, Delgado KH et al (2011) Steam reforming of methane over nickel: development of a multi-step surface reaction mechanism. Top Catal 54:845–858. https://doi.org/10.1007/s11244-011-9702-1
Takano A, Tagawa T, Goto S (1994) Carbon dioxide reforming of methane on supported nickel catalysts. J Chem Eng Jpn 27(6):727–731
Rostrup-Nielsen JR (1973) Activity of nickel catalysts for steam reforming of hydrocarbons. J Catal 31:173–199
Mhadeshwar AB, Vlachos DGJ (2005) Hierarchical multiscale mechanism development for methane partial oxidation and reforming and for thermal decomposition of oxygenates on Rh. J Phys Chem B 109(35):16819–16835. https://doi.org/10.1021/jp052479t
Deutschmann O, Schmidt L (1998) Modeling the partial oxidation of methane in a short-contact-time reactor. Am Inst Chem Eng AIChE 44:2465–2477
Schwiedernoch R, Tischer S, Correa C et al (2003) Experimental and numerical study of the transient behavior of a catalytic partial oxidation monolith. Chem Eng Sci 58(3):633–642. https://doi.org/10.1016/S0009-2509(02)00589-4
Liu M, Dana AG, Johnson MS et al (2021) Reaction mechanism generator v3.0: advances in automatic mechanism generation. J Chem Inf Model 61:2686–2696. https://doi.org/10.1021/acs.jcim.0c01480
Rakhi, Günther V, Richter J et al (2022) Steam reforming of methane over nickel catalyst using a one-dimensional model. Int J Environ Sci 5(1):1–32
LOGEsoft (2008) v1.08. www.logesoft.com
Aslanjan J, Klauer C, Perlman C et al (2017) Simulation of a Three-Way Catalyst Using Transient Single and Multi-Channel Models. SAE Technical Paper 2017-01-0966. http://papers.sae.org/2017-01-0966
Fröjd K, Mauss F (2011) A three-parameter transient 1D catalyst model. SAE Int J Engines 4(1):1747–1763. https://doi.org/10.4271/2011-01-1306
Müller K, Rachow F, Günther V et al (2019) Methanation of coke oven gas with nickel-based catalysts. Int J Environ Sci 4:73–79
Incopera F, DeWitt D, Bergman T et al (2006) Fundamentals of heat and mass transfer 6E. Wiley, Hoboken
Ramanathan K, Balakotaiah V, West D (2003) Light-off criterion and transient analysis of catalytic monoliths. Chem Eng Sci 58:1381–1405. https://doi.org/10.1016/S0009-2509(02)00679-6
Tischer S, Deutschmann O (2005) Recent advances in numerical modeling of catalytic monolith reactors. Catal Today 105:407–413. https://doi.org/10.1016/j.cattod.2005.06.061
Acknowledgements
Financial support by the federal ministry of education and research (Bundesministerium für Bildung und Forschung, BMBF) under the Grant Number 03SF0693A of the collaborative research project “Energie-Innovationszentrum” is gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rakhi, Shrestha, K.P., Günther, V. et al. Kinetically consistent detailed surface reaction mechanism for steam reforming of methane over nickel catalyst. Reac Kinet Mech Cat 135, 3059–3083 (2022). https://doi.org/10.1007/s11144-022-02314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02314-7