Abstract—
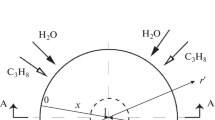
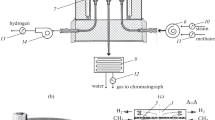
A model of steam methane reforming in a catalytic reactor has been developed the working part of which is two cylindrical chambers separated by a membranous wall. The upper chamber is evacuated while the lower one is maintained at atmospheric pressure. With a uniform supply of raw materials along the outer perimeter of the lower chamber, the problem is reduced to finding the average flows of CH4, H2O, CO2, CO, and H2 from the solution of a system of five nonlinear ordinary differential equations of the first order. The calculations were carried out for a Pd–6% Ru membrane in the temperature range of 673 K < T < 973 K at a steam/methane inlet flow ratio of 3 and a feed rate of 1800–9600 L/h. As a result of comparing the calculations with experimental data, a theoretical substantiation of the main regularities of the process observed in practice was obtained.






Similar content being viewed by others
REFERENCES
Ramachandran, R. and Menon, R.K., An overview of industrial uses of hydrogen, Int. J. Hydrogen Energy, 1998, vol. 23, no. 7, pp. 593–598. https://doi.org/10.1016/S0360-3199(97)00112-2
Kirillov, V.A., Meshcheryakov, V.D., Brizitskii, O.F., and Terent’ev, V.Ya., Analysis of a power system based on low-temperature fuel cells and a fuel processor with a membrane hydrogen separator, Theor. Found. Chem. Eng., 2010, vol. 44, no. 3, pp. 227–235. https://doi.org/10.1134/S0040579510030012
Schädel, B.T., Duisberg, M., and Deutschmann, O., Steam reforming of methane, ethane, propane, butane, and natural gas over a rhodium-based catalyst, Catal. Today, 2009, vol. 142, p. 42.
Butcher, H., Quenzel, C.J.E., Breziner, L., Mettes, J., Wilhite, B.A., and Bassard, P., Design of an annular microchannel reactor (AMR) for hydrogen and/or syngas production via steam reforming, Int. J. Hydrogen Energy, 2014, vol. 39, no. 31, p. 18046.
Shigarov, A.B., Meshcheryakov, V.D., and Kirillov, V.A., Use of Pd membranes in catalytic reactors for steam methane reforming for pure hydrogen production, Theor. Found. Chem. Eng., 2011, vol. 45, no. 5, p. 595. https://doi.org/10.1134/S0040579511050356
Gryaznov, V.M., Catalysis on membranes with selective permeability, Dokl. Akad. Nauk SSSR, 1969, vol. 189, p. 794.
Gryaznov, V.M., Hydrogen permeable palladium membrane catalyst. An aid to the efficient production of ultra pure chemicals and pharmaceuticals, Platinum Met. Rev., 1986, vol. 36, p. 68.
Yun, S. and Oyama, S.T., Correlations in palladium membranes for hydrogen separation: A review, J. Membr. Sci., 2011, vol. 375, nos. 1–2, pp. 28–45. https://doi.org/10.1016/j.memsci.2011.03.057
Orekhova, N.V., Kustov, L.M., Kucherov, A.V., Finashina, E.D., Ermilova, M.M., and Yaroslavtsev, A.B., Membrane catalytic systems for C2–C4 alkane conversion, Nanotechnol. Russ., 2012, vol. 7, pp. 560–574. https://doi.org/10.1134/S1995078012060109
Takao, K., Yoichi, I., Takaya, I., Hisataka, Y., Hiroyuki, T., Hideaki, H., Yasuhiro, T., and Masaya, I., Performance evaluation of membrane on catalyst module for hydrogen production from natural gas, Int. J. Hydrogen Energy, 2013, vol. 38, p. 6079.
Li, P., Wang, Zh., Qiao, Zh., Liu, Y., Cao, X., Li, W., Wang, I., and Wang, Sh., Recent developments in membranes for efficient hydrogen purification, J. Membr. Sci., 2015, vol. 495, p. 130.
Anzelmo, B., Wilcox, I., and Liguori, S., Hydrogen production via natural gas steam reforming in Pd–Au membrane reactor. Comparison between methane and natural gas steam reactions, J. Membr. Sci., 2018, vol. 568, p. 113.
Didenko, L.P., Sementsova, L.A., Chizhov, P.E., and Dorofeeva, T.V., Influence of the propane content in methane on the parameters of steam reforming of a mixture in a membrane reactor with the industrial nickel catalyst and Pd–Ru alloy foil, Int. J. Hydrogen Energy, 2019, vol. 44, p. 26396.
Babak, V.N., Didenko, L.P., and Zakiev, S.E., Hydrogen transport through a membrane module based on a palladium foil, Theor. Found. Chem. Eng., 2013, vol. 47, no. 6, pp. 719–729. https://doi.org/10.1134/S004057951306002X
Boeltken, T., Wunsch, A., Gietzelt, T., Pfeifer, P., and Dittmeyer, R., Ultra-compact microstructured methane steam reforming with integrated palladium membrane for on-site production of pure hydrogen: Experimental demonstration, Int. J. Hydrogen Energy, 2014, vol. 30, p. 18058.
Didenko, L.P., Savchenko, V.I., Sementsova, L.A., and Bikov, L.A., Hydrogen flux through the membrane based on the Pd–In–Ru foil, Int. J. Hydrogen Energy, 2016, vol. 41, p. 307.
Babak, V.N., Didenko, L.P., Kvurt, Yu.P., and Sementsova, L.A., Studying the operation of a membrane module based on palladium foil at high temperatures, Theor. Found. Chem. Eng., 2018, vol. 52, no. 2, pp. 181–194. https://doi.org/10.1134/S004057951802001X
Slovetsky, D.I. and Chistov, E.M., RF Patent 2416460, 2011.
Burkhanov, G.S., Gorina, N.B., Kolchugina, N.B., Roshan, N.R., Slovetsky, D.I., and Chistov, E.M., Palladium-based alloy membranes for separation of high purity hydrogen from hydrogen-containing gas mixtures, Platinum Met. Rev., 2011, vol. 55, no. 1, pp. 3–12. https://doi.org/10.1595/147106711x540346
Tong, H.D., Berenschot, J.W.E., de Boer, M.J., Gardeniers, J.G.E., Wensink, H., Jansen, H.V., Nijdam, W., Elwenspoek, M.C., Gielens, F.C., and van Rijn, C.J.M., Microfabrication of palladium-silver alloy membranes for hydrogen separation, J. Microelectromech. Syst., 2003, vol. 12, no. 5, pp. 622–629. https://doi.org/10.1109/JMEMS.2003.818458
Tong, J., Shirai, R., Kashima, Y., and Matsumura, Y., Preparation of a pinhole-free Pd–Ag membrane on a porous metal support for pure hydrogen separation, J. Membr. Sci., 2005, vol. 260, nos. 1–2, pp. 84–89. https://doi.org/10.1016/j.memsci.2005.03.039
Mazali, I.O., Filho, A.G.S., Viana, B.C., Filho, J.M., and Alves, O.L., Size-controllable synthesis of nanosized-TiO2 anatase using porous Vycor glass as template, J. Nanopart. Res., 2006, vol. 8, p. 141. https://doi.org/10.1007/s11051-005-9003-3
Huang, Y. and Dittmeyer, R., Preparation of thin palladium membranes on a porous support with rough surface, J. Membr. Sci., 2007, vol. 302, nos. 1–2, pp. 160–170. https://doi.org/10.1016/j.memsci.2007.06.040
Pizzi, D., Worth, R., Baschetti, M.G., Sarti, G.C., and Noda, K., Hydrogen permeability of 2.5 μm palladium–silver membranes deposited on ceramic supports, J. Membr. Sci., 2008, vol. 325, no. 1, pp. 446–453. https://doi.org/10.1016/j.memsci.2008.08.020
Gade, S.K., Thoen, P.M., and Way, J.D., Unsupported palladium alloy foil membranes fabricated by electroless plating, J. Membr. Sci., 2008, vol. 316, nos. 1–2, pp. 112–118. https://doi.org/10.1016/j.memsci.2007.08.022
Zhang, X., Xiong, G., and Yang, W., A modified electroless plating technique for thin dense palladium composite membranes with enhanced stability, J. Membr. Sci., 2008, vol. 314, nos. 1–2, pp. 226–237. https://doi.org/10.1016/j.memsci.2008.01.051
Bayati, B., Bayat, Y., Charchi, N., Ejtemaei, M., Babaluo, A.A., Haghighi, M., and Drioli, E., Preparation of crack-free nanocomposite ceramic membrane intermediate layers on α-alumina tubular supports, Sep. Sci. Technol., 2013, vol. 48, no. 13, pp. 1930–1940. https://doi.org/10.1080/01496395.2013.786728
Calles, J.A., Sanz, R., Alique, D., Furones, L., Marín, P., and Ordoñez, S., Influence of the selective layer morphology on the permeation properties for Pd-PSS composite membranes prepared by electroless pore-plating: Experimental and modeling study, Sep. Purif. Technol., 2018, vol. 194, pp. 10–18. https://doi.org/10.1016/j.seppur.2017.11.014
Xu, J. and Froment, G.F., Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics, AIChE J., 1989, vol. 35, no. 1, pp. 88–96. https://doi.org/10.1002/aic.690350109
Lin, Y.M., Liu, Sh.I., Chuang, Ch.H., and Chu, Y.T., Effect of incipient removal of hydrogen through palladium membrane on the conversion of methane steam reforming: Experimental and modeling, Catal. Today, 2003, vol. 82, no. 1, p. 127.
Falko, M. and Marrelli, P.L., Heat transfer and hydrogen permeability in modelling industrial membrane reactors for methane steam reforming, Int. J. Hydrogen Energy, 2007, vol. 32, p. 2094.
Krylov, O.V., Geterogennyi kataliz (Heterogeneous Catalysis), Moscow: Akademkniga, 2004.
Oliveria, E.L.G., Grande, C.A., and Rodrignes, A.E., Steam methane reforming in a Ni/Al2O3 catalyst: Kinetics and diffusional limitations in extrudates, Can. J. Chem. Eng., 2009, vol. 87, p. 945.
Demidovich, B.P., Maron, I.A., and Shuvalov, E.Z., Chislennye metody analiza (Numerical Methods of Analysis), Moscow: Gos. Izd. Fiz.-Mat. Lit., 1963.
Funding
This study was financially supported by the Russian Foundation for Basic Research (project no. 13-03-12419).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Babak, V.N., Didenko, L.P., Kvurt, Y.P. et al. Simulation of Steam Methane Reforming in a Membrane Reactor with a Nickel Catalyst and a Palladium Alloy Foil. Theor Found Chem Eng 55, 390–402 (2021). https://doi.org/10.1134/S0040579521030027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579521030027