Abstract
The palladium-catalyzed oxidative carbonylation of phenylacetylene (PCPOC reaction) is known to exhibit autonomous oscillations in a variety of parameters including pH, redox potential and heat of reaction. Previous studies report that external addition of both an oxidizing agent and water is required to allow production/consumption of H+ in an oscillatory fashion. In this work we show that solutions of PdI2/KI dried over 3 Å molecular sieves can catalyze phenylacetylene carbonylation in the absence of an external oxidizing agent. Oscillations are readily achieved in this considerably simplified PCPOC system. Palladium-catalyzed carbonylation of methanol generates HI as a by-product, which can react with excess KI and methanol producing methyl iodide and water in situ. Methyl iodide can also undergo decomposition, generating iodine as an internally formed oxidizing agent, which accounts for recorded catalyst regeneration. Oscillations are observed in the pH range 1.5–3.5 commencing, at the earliest, 70 min after introduction of phenylacetylene. Oscillations vary in period between 1 and 3.5 h and last for several days. The onset of oscillations may be delayed via portion-wise addition of the phenylacetylene substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, chemical systems that exhibit rhythmical oscillations have progressed from fascinating, but obscure phenomena to a valuable driving force for responsive and self-actuating materials [1, 2]. As our understanding of the mechanisms underlying these complex systems continues to develop, so does the potential to exploit key characteristics such as autonomous changes in pH. A wide variety of systems are now known to act as chemical oscillators including variations of the well-known Belousov-Zhabotinsky and Briggs-Rauscher reactions [3]. pH oscillators, defined as systems in which changes in H+ concentration are the driving force behind oscillatory behavior [4], rely on autocatalytic generation of H+ and corresponding reactions that consume H+. Such reactions usually involve the oxidation of organic substrates, (e.g. malonic acid), by a variety of oxidants including O2, H2O2, BrO3 − and IO3 −, forming CO2, water etc. Each process is catalyzed by transition metal ions and complexes such as Ce(IV)/Ce(III), Ru(III)/Ru(II), Fe(III)/Fe(II) or Mn(III)/(Mn(II) [3].
An alternative class of chemical oscillator is that in which complex organic molecules are synthesized from simple building blocks such as carbon monoxide, alcohols and acetylenes. Mechanistic pathways are present that both produce and consume H+ leading to periodic oscillations in pH. One such example is the palladium-catalyzed oxidative carbonylation of acetylenes to esters and carboxylic acids [5, 6]. The oxidative carbonylation of phenylacetylene (PCPOC reaction) using PdI2, KI, O2 and NaOAc in methanol was shown to display oscillations in pH, redox potential and the rate of consumption of a CO–O2 gas mixture [6, 7]. Substrates such as methylacetylene and 2-methyl-3-butyn-2-ol display similar oscillatory behavior [8]. More recent studies of the PCPOC reaction using power compensation calorimetry confirm synchronized oscillations in reaction exothermicity and pH. A ‘staircase’ of exothermic pulses of up to 600 J per oscillation coincides with corresponding falls in pH [9].
Interestingly, the oxidative carbonylation of phenylacetylene has been shown to act in both oscillatory and non-oscillatory modes with significant differences observed in both conversion and product selectivity [10, 11]. In the case of non-oscillatory oxidative carbonylation, product formation is unhindered from the start of the reaction and results in a mixture of products including: E-3-phenylacrylic acid methyl ester, 1; 2-phenylacrylic acid methyl ester, 2; Z-2-phenylbut-2-enedioic acid dimethyl ester, 3; E-3-phenylacrylic acid methyl ester, 4; 3-phenyl-5H-furan-2-one, 5; 3-phenylfuran-2,5-dione, 6 and Z-2-phenyl-2-butenedioic acid, 7, Fig. 1 [10]. When operating in an oscillatory regime, product formation is suppressed pending the onset of oscillations, upon which 3 is formed with greater than 75 % selectivity. Minor products are 4, 6, 7 and 5,5-dimethoxy-3-phenyl-furan-2(5)-one, 8 [11].
Reaction products observed during the palladium-catalyzed oxidative carbonylation of phenylacetylene. Operation in a non-oscillatory mode yields mixtures of 1, 2, 3, 4, 5, 6 and 7. 6/7 are not readily distinguished using GC–MS [12]. In contrast, operation in an oscillatory regime yields 3 as the major product with 4, 6/7 and 8 observed in smaller quantities
The process of oxidative carbonylation in a non-oscillatory regime has been studied in detail by Gabriele et al. [13]. In order for the reaction to be catalytic rather than stoichiometric, an external oxidant (such as quinones, copper(II), oxygen or a combination of these) is required to drive reoxidation of the metal center. Traces of water may limit catalytic activity due in part to competitive oxidation of CO to CO2 [13, 14]. Oxidation of CO can proceed stoichiometrically, generating HI as a by-product, or catalytically, in the presence of an oxidant such as O2, reducing both selectivity and conversion [13].
Water has been reported to form in a 1:1 ratio to products in the oscillatory carbonylation of phenylacetylene. However, the authors also add water prior to commencing the reaction (~0.2 M initial concentration) as it is believed to be crucial in the formation of an intermediate HPdI species which contributes to oscillatory behavior [6]. The formation of diesters in an oscillatory regime is reported to be catalyzed by Pd(I)-based species (e.g. Pd2I2 or Pd2I4(CO) 2−2 ), even in the presence of oxygen, with Pd2I2 formed via reaction of HPdI with either PdI2 or O2 (generating HI or H2O2 as by-products, respectively) [14]. The presence of an external oxidizing agent was also understood to be necessary for both oscillatory and non-oscillatory carbonylation processes to proceed catalytically [14].
In this work we demonstrate that solutions of PdI2/KI in methanol, dried over 3 Å molecular sieves, can catalyze the oxidative carbonylation of phenylacetylene in an oscillatory mode. In addition, it is revealed that this oscillatory system does not require an external oxidizing agent in order for catalyst recycling to occur. Furthermore, oscillatory behavior is recorded using considerably lower concentrations of both PdI2 (approximately fivefold) and phenylacetylene (approximately 40–80-fold) compared to those reported previously [9–11, 17]. As a result, the transparency of the reaction solution is significantly increased.
Experimental
Materials and methods
All materials were purchased from Sigma Aldrich and used without further purification unless otherwise stated. Catalyst solutions were prepared by dissolving palladium(II) iodide (401 mg, 1.1 mmol) and potassium iodide (24.93 g, 150 mmol) in HPLC grade methanol (300 mL). The mixture was stirred at ambient temperature for 48 h and the resulting solution filtered through a 0.2 μm Millipore membrane filter to remove residual PdI2 (57 mg). The concentration of the resulting catalyst solution was then adjusted using additional methanol and the solution dried (minimum of 24 h) and stored over 3 Å molecular sieves prior to use. Catalyst solutions were then either used neat or diluted to working concentrations using pre-dried HPLC grade methanol (stored over 3 Å molecular sieves for a minimum of 24 h prior to use). Catalyst solutions stored over molecular sieves are referred to as pre-dried rather than dry within the text as water concentrations are very low, but non-zero [15]. Previous studies report vastly different behavior when comparing HPLC grade solutions to those stored over molecular sieves [12].
All experiments were conducted at room temperature with no external temperature control. However, the temperature was recorded during each reaction. For simplicity, pH values were measured using an aqueous pH electrode calibrated using aqueous buffers (pH 2, 7 and 10). Due to the non-aqueous nature of the reaction media (methanol) and differing junction potentials during calibration and sample measurement, absolute values of hydrogen ion activities are not obtained. Instead an approximation is acquired using adjusted pH values (pHadj), which provides a useful comparison of relative acidities. In methanol, pHadj is approximated by adding 2.3 pH units to the measured value of pH (pHapp) and hydrogen ion concentrations subsequently estimated using 10−(pHapp + 2.3) [16]. The formation of water during the PCPOC reaction could in theory change the solvent composition and affect pKa values. However, the amounts expected to be produced are well below the 1 % v/v level suggested to have an effect and for this reason are discounted [16].
Sampling procedures
Samples were filtered through silica to remove any solid palladium species and transferred to GC vials. The solutions were then analyzed using GC–MS without further dilution. Analysis of samples was carried out using a Varian Saturn 2000 GC–MS fitted with a Varian VF-5 ms column. The same GC method was employed for all samples: injector temperature 150 °C, initial oven temperature 100 °C, followed by five successive ramps (1 min at 20 °C min−1 then hold for 5 min) up to 195 °C, which was held for 8 min.
Oscillatory runs
Two semi-batch runs are reported as examples of the palladium-catalyzed carbonylation of phenylacetylene operating in an oscillatory mode. Pre-dried solutions of PdI2/KI were used as described in Table 1 and a stable baseline pH value was obtained for approximately 5 min prior to purging with CO. Exact baseline values, whilst dependent upon [PdI2], were stable within any given experiment. Phenylacetylene was then added in aliquots as described in Table 1. CO purging was maintained throughout the course of the reaction resulting in a positive pressure of CO in the closed but not hermetically sealed reaction vessels.
Run 1 was conducted on a small scale (17 mL of pre-dried PdI2/KI solution, Table 1) in a 25 mL sample vial and was monitored using a HEL MicroNOTE system. After determination of a stable baseline pH, the PdI2/KI solution was purged with CO (10 mL min−1). Phenylacetylene (1 μL) was added 16 min after the onset of CO purging, with two further 1 μL aliquots of phenylacetylene added 62 and 180 min later, giving a total concentration of phenylacetylene of 1.6 mM, Table 1.
Run 2 was conducted on a larger scale (90 mL of pre-dried KI/PdI2 solution) in order to allow for sampling. The reaction was carried out in a jacketed glass vessel with ports to accommodate pH and temperature probes, CO inlet and allow easy removal of samples. A new batch of catalyst solution was prepared (concentration as described in Table 1) and the experiment was monitored using a different pH probe and different equipment (HEL Simular). Due to the limitations of the mass flow controller, a higher CO flow rate of 15 mL min−1 was used. Prior to commencement of pH monitoring, naphthalene (231 mg, 1.8 mmol, 0.02 M) was dissolved in the catalyst (PdI2/KI) solution to act as an internal standard. After determining the baseline pH, the PdI2/KI/naphthalene solution was purged with CO (15 min) and treated with phenylacetylene (29.5 μL), giving a total phenylacetylene concentration of 3 mM. CO purging was maintained throughout the experiment. Compared to Run 1, higher concentrations of PdI2, KI and phenylacetylene were used with phenylacetylene added as a single aliquot.
Initial pH drop upon CO purging
Studies relating catalyst concentration and the initial pH drop upon CO purging of pre-dried catalyst solution were carried out using HEL MicroNOTE connected to a pH electrode and Pt100 temperature probe. In each case, the pH electrode was immersed in the catalyst solution and a baseline pH reading obtained over a 5 min period. Each solution was then purged for 15 min with CO (10 mL min−1) and the pH monitored.
Formation of methyl iodide
In order to investigate the formation of methyl iodide as a function of time, the pH of a pre-dried solution of PdI2/KI (25 mL, 2.68 mM PdI2), containing naphthalene (0.002 M) as an internal standard, was monitored for 5 min using HEL MicroNOTE before purging with CO (10 mL min−1). Samples were taken at various intervals over a 5 h period, filtered and analyzed using GC–MS. Molecular sieves (3 Å, 350 mg) were added 259 min into the experiment to assess the response to the removal of water.
Results and discussion
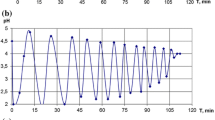
The oscillatory pH behavior recorded in Run 1 is given in Fig. 2. Corresponding hydrogen ion concentrations were approximated from adjusted pH values (pHadj), Fig. 2c [16]. Phenylacetylene was added portion-wise to a pre-dried solution of PdI2/KI in methanol purged continuously with CO. Lower catalyst concentrations, compared to those reported previously [6, 9], give a solution with increased transparency. In addition, the use of pre-dissolved catalyst solutions, as opposed to solid reagents used previously, allows exact initial concentrations of PdI2 to be determined.
Run 1: changes in pH upon palladium-catalyzed oxidative carbonylation of phenylacetylene operating in an oscillatory mode. a Complete reaction. b Behavior during initial stages of the reaction. Phenylacetylene (1 μL, molar equivalent relative to [PdI2]) was added at points, 1, 2 and 3, respectively. c Oscillatory region showing changes in pH and corresponding [H+]adj as a function of time. Reaction volume = 17 mL; [PdI2] = 5.4 × 10−4 M; [KI] = 0.08 M; CO = 10 mL min−1; [PhAc]total = 1.6 × 10−3 M
A steady fall in pH is observed upon addition of 1 molar equivalent of phenylacetylene (relative to the concentration of PdI2). The rate at which the pH falls is accelerated by addition of a second equivalent of phenylacetylene. The observed pH reaches a minimum 62 min after the second addition of phenylacetylene, Fig. 2b. The pH then steadily rises until a further aliquot of phenylacetylene is introduced. After a 10 min induction period, a sharp drop in pH is observed, reaching a minimum after 20 min. The pH then exhibits a steady rise over a period of 600 min after which stable oscillations in pH commence. The induction period observed prior to entering the oscillatory region is considerably shorter than the 2,000 min recorded previously at similar temperatures [17]. Temkin and co-workers [6, 8] were able to achieve oscillations in pH within minutes of starting a PCPOC reaction, however they reported an experimental setup using mixed gases (CO/O2) and higher reaction temperatures (40 °C). The behavior reported in the present study was observed at higher catalyst loadings, using pre-dried solutions and without an external supply of oxygen to recycle the catalyst.
Oscillations vary in period between 96 and 216 min, with a maximum amplitude of 1.2 pH units. The change in the concentration of hydrogen ions within a given oscillation, Δ[H+]adj, is in the range 1–5 × 10−5 mol dm−3, with an average of 2 × 10−5 mol dm−3 per oscillation. The time for the pH to fall from its maximum to minimum value within any given oscillation is in the range 22–46 min. Previous studies have shown that the time required for pH to fall within an oscillation is constant at a given temperature, with the time approximately doubling with a reduction of 10° [17]. This behavior indicated a pseudo-zeroth order process, in which the reaction rate is independent of reagent concentrations, e.g. these are considered to be in excess. In the case of HPLC grade methanol used in previous studies, water is present in excess whereas for the pre-dried solutions reported here this is no longer the case.
Run 2 (Fig. 3) serves to illustrate that oscillatory behavior is both repeatable and also observed under different experimental conditions. During Run 2, stable oscillations begin 70 min after addition of phenylacetylene with the peak of the first oscillation appearing 77 min later, a considerably shorter induction time than observed for Run 1. Oscillations continue in a relatively stable manner for ~1,600 min (16 cycles) after which they start to become more irregular in shape. After a period of 2,400 min oscillatory behavior becomes increasingly erratic but changes in pH were still observed until the reaction was terminated (4,500 min). Oscillations have higher amplitudes and shorter periods (mean values of 0.9 pH units and 100 min, respectively) than for Run 1. In addition, the time recorded for the pH to fall from its maximum to minimum value is also shorter, averaging 19 min. A summary of the oscillatory behavior observed in Runs 1 and 2 is given in Table 2.
Run 2: changes in pH upon palladium-catalyzed oxidative carbonylation of phenylacetylene operating in an oscillatory mode. a Complete reaction. b Oscillatory region showing changes in pH and corresponding [H+]adj as a function of time. Reaction volume = 90 mL; [PdI2] = 6.4 × 10−4 M; [KI] = 0.150 M; CO = 15 mL min−1; [PhAc]total = 3.0 × 10−3 M, added 15 min after onset of CO purging
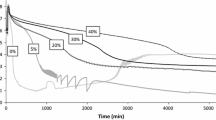
During the course of Run 2, samples of the reaction mixture were analyzed using GC–MS to determine the concentration of phenylacetylene and identify the products. Conversion of phenylacetylene proceeds to 58 %, Fig. 4a, forming a mixture of 3, 4, 8 and methyl iodide, Fig. 4b. Analytical standards of 4 and 8 were unavailable hence their GC–MS response and absolute concentrations were not calculated. Instead a combined (total) concentration of 4/8 is reported, Fig. 4b. The cecorded phenylacetylene conversion, in conjunction with a PdI2 loading of 21.3 %, suggests a catalytic rather than stoichiometric process. Pre-dried solutions of PdI2 and KI can thus catalyze the PCPOC reaction in the absence of an external oxygen supply.
Distribution of phenylacetylene and products in the PCPOC reaction. a Oscillatory pH behavior with corresponding conversion of phenylacetylene. b Concentrations of phenylacetylene and observed products during the PCPOC reaction. Combined concentration of 4 and 8 calculated from GC–MS. Reaction volume = 90 mL; [PdI2] = 6.4 × 10−4 M; [KI] = 0.150 M; CO = 15 mL min−1; [PhAc]total = 3.0 × 10−3 M, added 15 min after onset of CO purging. [Naphthalene] = 0.02 M
Observation of methyl iodide within the pre-dried PCPOC system suggests a possible mechanism to account for both the limited pH drop upon CO purging and also formation of water in situ. PdI2 shows limited solubility in alcohol solvents. However, the soluble PdI4 2− anion is formed and stabilized in solution upon introduction of excess KI [18]. Gabriele et al. [19] have previously reported the oxidative carbonylation of a variety of alkynes using PdI2/KI and palladium iodide-thiourea (tu) catalysts with a thiourea/palladium ratio of 3 or 3.5:1. Analogous PdCl2, PdBr2 and thiourea systems such as [Pd(tu)2I2], [Pd(tu)4]I2 or [Pd(tu)4](BF4)2] are poor or ineffective catalysts. The degree of dissociation within palladium catalysts has been highlighted as essential to their activity in carbonylation; PdI4 2− is likely to be at least partially dissociated in methanol solution [18, 19]. The mechanism of phenylacetylene carbonylation, as reported by Gabriele [18], is given in Eqs. 1–4 (Scheme 1). HI is generated as a by-product accounting for the observed falls in pH [13]. The presence of methyl iodide suggests an equilibrium reaction between methanol and HI, generating methyl iodide and water (Eq. 5). This theory is further supported by larger pH drops reported when non-dried catalyst solutions are used, which are suppressed and delayed in pre-dried solutions [12]. The presence of water may initiate a cascade of reactions, including formation of Pd(I) species, which are thought to be active catalysts in the oscillatory process [7, 14]. Furthermore, unstable methyl iodide that decomposes to iodine and ethane, can account for formation of an oxidizing agent (i.e. iodine) in situ, Eq. 6 [20], and explain recorded catalyst regeneration, Eq. 7 [21].
In order to validate the mechanism proposed in Scheme 1 and probe the behavior of the catalyst system in the absence of water, a series of pre-dried catalyst solutions of PdI2/KI of differing concentration were prepared. A baseline pH reading was obtained for each solution over a five minute period. The exact value of apparent pH, pHapp, varies between runs, increasing from pH 7 to 9 with decreasing concentration of PdI2. Upon purging with carbon monoxide, a rapid drop in pH is observed, stabilizing after 5–10 min purging. Discussion in terms of pH fall is not particularly instructive given the different starting pH values. The change in concentration of hydrogen ions upon CO purging, Δ[H+], is calculated as [H+]adj, (time t) − [H+]adj, baseline and relates to the amount of HI produced, Fig. 5. Δ[H+] upon CO purging increases as a function of [PdI2], suggesting that the rate of HI formation is a function of [PdI2] (or more specifically the [PdI4]2− anion) which is in agreement with Eq. 1.
To examine the processes limiting the formation of hydrogen ions, samples were taken at various intervals from a CO purged solution of PdI2/KI and analyzed by GC–MS. The pH of the solution was monitored throughout and corresponding [H+]adj approximated, Fig. 6. CO purging induces a rapid increase in [H+], which then slows and eventually stabilizes around 1 × 10−5 M. Addition of molecular sieves to the reaction mixture leads to a sharp drop in [H+].
Analysis of the CO-purged catalyst solutions by GC–MS indicates the presence of methyl iodide, the concentration of which varies during the course of the reaction. Due to the low concentrations of methyl iodide produced (<1 × 10−4 M), its volatile nature and low GC response factor satisfactory quantification was not possible. However qualitative trends were noted. For example addition of molecular sieves leads to a large drop in [H+] and increase in [CH3I]. This behavior is consistent with removal of water, consumption of HI and a shift of the equilibrium in Eq. 5 to the right. Methyl iodide production is limited by the concentration of HI in the initial stages of the reaction, Eq. 1. Synthesis of methyl iodide in methanol is known to proceed either via direct reaction of methanol with a strong acid such as HI or via an acid-catalyzed pathway in which methanol reacts with an iodide salt [22, 23]. In the PCPOC system, KI is likely to play a part in the formation of methyl iodide, due to the large excess coupled with the relatively low concentrations of HI. The reaction is also a reversible process. Carbonylation of methyl iodide and methanol to methyl acetate is known to be catalyzed by palladium(II) salts in the presence of excess iodide. However, the reaction requires high temperatures and pressures of CO [24]. Methyl acetate was not detected so consumption of methyl iodide via such a pathway is thought to be unlikely.
Conclusions
We thus propose that the oscillatory pH behavior observed in pre-dried solutions of PdI2/KI is a result of the in situ formation of methyl iodide, water and iodine. Methyl iodide and water are generated reversibly in situ via reaction of HI, a by-product of palladium-catalyzed carbonylation of methanol in the presence of KI. Iodine, formed upon decomposition of methyl iodide, provides an in situ oxidizing agent, allowing for regeneration of the PdI2 catalyst and the observed catalytic nature of this oscillatory carbonylation process. Stable oscillations of up to 1.5 pH units are observed for several days, the onset of which can be delayed via portion-wise introduction of the phenylacetylene substrate.
References
Yoshida R (2010) Adv Mater 22:3463–3483
Crook CJ, Smith A, Jones RAL, Ryan AJ (2002) PCCP 4:1367–1369
Epstein RI, Pojman JA (1998) An introduction to nonlinear chemical dynamics: oscillations waves. Patterns and chaos. Oxford University Press, New York
Rábai G, Orbán M, Epstein IR (1990) Acc Chem Res 23:257–263
Shulykovskii GM, Temkin ON, Bikanova NV, Nirkova AN (1985) In chemical kinetics and catalysis: kinetic models of liquid phase reactions. Institute of Chemical Physics, Chernogolovka, p 112 (in Russian)
Malashkevich AV, Bruk LG, Temkin ON (1997) J Phys Chem A 101:9825–9827
Gorodskii SN, Zakharov AN, Kulik AV, Bruk LG (2001) Temkin ON Kinet Catal 42:251–263
Gorodskii SN, Kalenova ES, Bruk LG, Temkin ON (2003) Russ Chem Bull Int Ed 52:1534–1543
Novakovic K, Grosjean C, Scott SK, Whiting A, Willis MJ, Wright AR (2007) Chem Phys Lett 435:142–147
Grosjean C, Novakovic K, Scott SK, Whiting A, Willis MJ, Wright AR (2008) J Mol Catal A Chem 284:33–39
Novakovic K, Grosjean C, Scott SK, Whiting A, Willis MJ, Wright AR (2008) PCCP 10:749–753
Parker J, Novakovic K (2013) Ind Eng Chem Res 52:2520–2527
Gabriele B, Salerno G, Costa M (2006) Top Organomet Chem 18:239–272
Temkin ON, Bruk LG (2003) Kinet Catal 44:601–617
Bradley D, Williams G, Lawton M (2010) J Org Chem 75:8351–8354
Porras SP, Kenndler E (2004) J Chromatogr A 1037:455–465
Novakovic K, Mukherjee A, Willis M, Wright AR, Scott SK (2009) PCCP 11:9044–9049
Gabriele B (1994) J Chem Soc Perkin Trans 1:83–87
Gabriele B, Salerno S, Costa M, Chiusoli GP (1995) J Organomet Chem 503:21–28
Harris GM, Willard JE (1954) J Am Chem Soc 76:4678–4687
Gabriele B, Mancuso R, Salerno G (2012) Eur J Org Chem 2012:6825–6839
Pickard RH, Kenyon J (1911) J Chem Soc 99:45–72
Stone H, Shechter H (1950) J Org Chem 15:491–495
Yang J, Haynes A, Maitlis PM (1999) Chem Comm 179–180
Acknowledgments
This work was supported by Engineering and Physical Sciences Research Council (EPSRC) Grant EP H003908/1. JP would like to acknowledge Professor Allen Wright for funding her Ph.D. studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Donlon, L., Parker, J. & Novakovic, K. Oscillatory carbonylation of phenylacetylene in the absence of externally supplied oxidant. Reac Kinet Mech Cat 112, 1–13 (2014). https://doi.org/10.1007/s11144-014-0685-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0685-3