Abstract
The PdI2 catalyzed oxidative carbonylation of phenylacetylene has been shown to produce pH oscillations over the temperature range 0-40 °C under semi-batch conditions. The decrease in reaction temperature resulted in an increase in the period and amplitude of the pH oscillations. In addition, reducing the reaction temperature enabled the observation of different phases in the oscillatory pH behavior confirming the ability of the reaction system to autonomously produce multiple clusters of oscillatory phases. Sets of pH oscillations were recorded at 0 and 10 °C. At temperatures of 0–20 °C, oscillations commenced as pH was falling during the initial stages of the reaction while, at 30 and 40 °C, the oscillations commenced once the pH started to increase after the initial drop. Oscillations in turbidity were observed at 20 and 30 °C. Experiments were conducted in an HEL Simular reaction calorimeter enabling precise temperature control. Operating at 0 °C reduced the reaction rate prolonging the duration of pH oscillations. The oscillatory pH behavior was recorded for 11 days without additional substrate at which point the run was terminated although the oscillations would likely have continued.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative carbonylation reactions are important C–C bond forming reactions that generate a number of products depending on the substrates, catalyst and reaction conditions [1]. Using a PdI2-KI catalyst with alkynes under mild conditions, high catalytic efficiencies have been achieved leading to a mixture of dicarbonylation products [2]. When phenylacetylene (PhAc) was used as the substrate in methanol (palladium-catalyzed phenylacetylene oxidative carbonylation, PCPOC), oscillations in pH, redox potential and gas uptake (CO and O2) were discovered [3–7]. Further study of this reaction using power compensation calorimetry found the oscillations in pH were accompanied by oscillations in heat of reaction, Qr [8]. The products of the reaction have been identified in both oscillatory and non-oscillatory modes and consist of mono- and di-esters, anhydrides, acids and heterocyclic compounds depending on the reaction conditions [9–11]. The products include methyl (E)-cinnamate; methyl atropate; dimethyl (2Z)-2-phenyl-2-butenedioate; dimethyl (2E)-2-phenyl-2-butenedioate and 5,5-dimethoxy-3-phenyl-2(5H)-furanone.
The period and amplitude of pH oscillations in the PCPOC reaction were shown to be temperature dependent when the reaction temperature was varied from 10–40 °C. Lower temperatures produced oscillations with a larger period and amplitude [12]. This work investigates the effect of reaction temperature further to ascertain if the observed trends continue at lower temperatures and are reproducible. In addition, this study records turbidity (a measure of the clarity of the solution due to suspended particles) during the reaction at each temperature to assist in mechanistic understanding of the oscillatory phenomena recorded in this reaction system. A study of the PCPOC reaction in oscillatory mode over the temperature range 0–40 °C was thus conducted using an HEL Simular reaction calorimeter to precisely control the reaction temperature following the procedure previously reported [12] with the inclusion of a turbidity probe. Additionally, naphthalene (Np) was added to the reaction system to act as an internal standard and enable monitoring of evaporative loss of solvent anticipated in lengthy experiments in this closed but not sealed reactor setup.
Experimental
The study was conducted using the HEL Simular, a reaction calorimeter that has the ability to record heat flow data whilst performing chemical reactions. The setup used consists of a 1 L double-jacketed glass reactor with an outer vacuum jacket and an inner oil jacket. Reactor temperature is monitored by a Pt100 temperature probe while internal heating is provided by a 150 W internal heater. The oil jacket temperature is controlled by a Julabo FP50-HD circulator with a working temperature range of −50 to 200 °C. The reactor is fitted with a combined pH electrode as well as a turbidity probe measuring relative turbidity by reflectance. This probe shines red light across a gap to a reflector. The intensity of the reflected light is measured and the difference is calculated as the turbidity measurement. If crystals/particles form in the solution the light is scattered and less light is reflected back to be detected resulting in a change in turbidity. The turbidity probe can, therefore, be used to monitor crystal formation. However, as it is a reflectance probe, it may also be affected by changes in the color of the liquid. If the reaction mixture changes color so that it absorbs more red light, then this will also be observed as a change in turbidity. Stirring is provided by an overhead pitched blade impeller. The reactor has a Brooks Smart series 5850S mass flow controller capable of dosing gases with flow rates from 0.003–30 L min−1, which was used to dose CO. There is an independent Cole-Parmer 16 Series mass flow controller with flow rates ranging from 5 to 500 mL min−1 that was used to control air flow. The installed WinISO software allows the HEL Simular to run completely unaided once it is set up. All data are automatically saved via the data logging software for later analysis using the proprietary HEL IQ software.
The experiments were conducted as follows: methanol (400 mL) was added to the HEL Simular reactor and heated to the required reaction temperature (0, 10, 20, 30 or 40 °C) while stirring at 250 rpm after which the palladium(II) iodide (434 mg) was added. After stirring at 250 rpm for 35 min, 113 mg of NaOAc and 37.392 g of KI were added along with 30 mL of methanol to wash down any solid remaining on the reactor walls. The stirrer speed was increased to 350 rpm to aid the dissolution of the KI. After a further 10 min, 1.154 g of Np was added followed by 20 mL of methanol. Five minutes after adding the Np, the stirrer speed was returned to 250 rpm and purging with CO and air at 50 mL min−1 commenced. Phenylacetylene (6.2 mL) was added to the reactor 20 min after purging began. Temperature, pH and turbidity were recorded throughout and additional methanol was added as necessary to compensate for evaporative loss of the solvent. All experiments were conducted using the power-compensation mode of the reaction calorimeter.
Results and discussion
The experiments in this study were conducted at temperatures of 0, 10, 20, 30 and 40 °C. The pH and turbidity recorded at each temperature are shown in Fig. 1. It can be noted that the experiments conducted at 0 and 10 °C exhibited several sets of pH oscillations. Only one set of pH oscillations was recorded at 20 and 30 °C although in the experiment conducted at 30 °C, prior to the occurrence of oscillations, unusual pH fluctuations were recorded around 1000 min. Interestingly, the experiment conducted at 40 °C showed 2 sets of oscillations. However, the second set of oscillations began at 5435 min (i.e. the end of the experiment) and seemed to be induced by the addition of methanol to the system, which was added to compensate for evaporative loss of solvent. The addition of methanol was not responsible for the occurrence of sets of oscillations in the experiments conducted at 0 and 10 °C. The experiments conducted at 0 and 10 °C were stopped before the oscillations had ceased due to time constraints. It is likely that more sets of oscillations would have been observed if the experiments were conducted for longer as the pH of the reaction solution was still in the oscillatory region when the experiments were stopped. This is in line with previous studies in which 3 sets of oscillations were reported at 10 °C during an experiment lasting for over 8000 min [12]. The duration of the pH oscillations recorded at 0 °C was over 16,000 min and is the longest reported so far for the PCPOC system.
The previous work, employing experiments at 10–50 °C, showed that the frequency and amplitude of the pH oscillations varies with temperature from 10–40 °C with higher temperatures producing smaller amplitude, higher frequency oscillations. As the temperature was decreased, the period and amplitude of the oscillations increased. No pH oscillations were observed at 50 °C [12]. A comparison of trends in the period and amplitude of the pH oscillations produced by the PCPOC reaction in this study and those reported previously are summarized in Tables 1 and 2.
The onset of oscillations (Table 2) occurred at different times in all of the experiments. Although the time of onset of the oscillations did not appear to be temperature dependent, it can be noted from Fig. 1 that the pH oscillations in the experiments conducted at 0–20 °C started when the pH was falling whereas at 30 and 40 °C the pH oscillations started when the pH had started rising following the initial fall. In general, the duration of the pH oscillations increased as the temperature decreased. The increase in the duration of the oscillations as the reaction temperature was decreased is likely a consequence of the reduction in reaction rate at lower temperatures. However, in the experiment conducted at 40 °C, the oscillations lasted for over 4000 min in this study compared to the 640 min in the 40 °C experiment conducted by Novakovic et al. [12]. This difference in duration of the pH oscillations at 40 °C was accompanied by a reduction in the maximum amplitude of the oscillations to 1/3 of that previously reported (Table 2). The difference in the experiments at 40 °C may be explained by variation in the granularity of PdI2 noted by Novakovic et al. when they had difficulties replicating the oscillatory behavior of their previous experiments at 40 °C [13]. If all of the PdI2 is not in solution this may introduce mass-transfer limitations by altering the concentration of catalyst available in solution and hence affecting the dynamics of the relevant reactions in the reaction network (e.g. involving the generation and consumption of [H+]) thereby altering the oscillatory pH behavior. At lower temperatures, the reaction rate is slower, as expected from the Arrhenius law, and therefore these mass transfer limitations may not be an issue. In terms of the period of oscillations the results are in agreement with those previously reported [12] i.e. the period of oscillations increased as reaction temperature decreased and this trend continued down to 0 °C with the actual values at each temperature being very similar to those found previously (see Tables 1, 2).
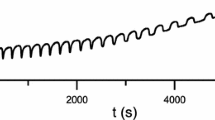
The pH oscillations at 0 and 10 °C behaved similarly. The initial pH oscillations were symmetrical in shape (Figs. 2a and 2c). As the pH oscillations at 0 and 10 °C increased in size, they became more shark-fin like (Figs. 2b and 2d).
The shape of the pH oscillations also changed during the course of the reaction at 20 °C. However, unlike at 0 and 10 °C, which produced symmetrical oscillations initially, the oscillations had a saw-tooth waveform when they started (Fig. 3a). The shape changed to the familiar shark-fin shape once the oscillations were established (Fig. 3b) and then became flatter and more symmetrical as they gradually ceased (Fig. 3c).
The general shape of each oscillation at 30 °C was initially similar to that found previously [12] but near the end of the experiment the shape of the oscillations changed (Fig. 4). Instead of the relaxation oscillations, which occurred for the majority of the reaction where pH increased gradually over approximately 20 min, slowed as it reached the local maximum, then dropped rapidly to the local minimum, the pH gradually increased to the local maximum over 15 min and then fell slowly in a linear fashion for 20–25 min before dropping rapidly to the local minimum.
At 40 °C the waveform was fairly symmetrical and did not change during the course of the reaction as shown in Fig. 5. This is in contrast to the shark fin-like waveform observed in the previous study at 40 °C [12]. It can also be noted from Figs. 5a and 5c that the addition of methanol to the reaction caused an increase in the period and amplitude of the pH oscillations and also restarted the pH oscillations once they had ceased (Fig. 5d). This is likely due to the addition of water to the system which is present in a concentration of <0.03 % in the HPLC grade methanol used.
The turbidity of the reaction mixture was also recorded. As relative turbidity was measured it is only an indication of the changes that occur during the reaction and cannot give an absolute measure of concentrations. The turbidity was measured in arbitrary units with a turbidity of 0 being assigned to the HPLC grade methanol used in the experiments (a clear, colorless liquid with no visible particles). Oscillations in turbidity have been reported during the carbonylation of alkyne-functionalized poly(ethylene glycol) (PEGA) at 20 °C [14]. The oscillations in turbidity using PEGA were out of phase with the oscillations in pH. The turbidity oscillations were attributed to changes in the oxidation state of the palladium during the course of the reaction [14]. The catalyst and substrate concentrations used in the PEGA experiments ([PdI2] = 4.05 × 10−5 mol dm−3, [PEGA] = 2.03 × 10−3 mol dm−3) were much lower than the concentrations used in this study ([PdI2] = 2.64 × 10−3 mol dm−3, [PEGA] = 0.124 mol dm−3), resulting in a more transparent reaction solution than the very dark solution studied here. The turbidity recorded during the oscillatory PCPOC runs is shown in Fig. 1.
At 0 °C the turbidity remained fairly constant at 32.5 for most of the experiment (Fig. 1). However, a drop in turbidity occurred at the same time as the final oscillation in each set of pH oscillations (Fig. 6). This suggests that the last pH oscillation coincided with either a drop in the amount of precipitate which then reformed or a change in the color of the solution. Both of which could indicate a change in oxidation state of the palladium.
Fig. 7 shows the turbidity recorded during the PCPOC reaction at 10 °C. The spikes at the beginning of the reaction were due to the addition of reagents to the reactor. After a large dip to a value of 23 occurring between 500 and 1200 min, the turbidity recovered and fluctuated around a value of 31 (Fig. 7). Similarly to the 0 °C run, a drop in turbidity (from 32.5 to 27.6) occurred at the same time as the final oscillation in the first set (3130–3270 min).
The turbidity recorded at 20 °C fluctuated around a value of 30 for most of the reaction (Fig. 1). However, at approximately 2200 min, the turbidity began to fluctuate more regularly until sustained oscillations were observed. The turbidity oscillations were out of phase with the pH oscillations (Fig. 8). At 2882 min a large drop in turbidity was recorded (from 32.3 to 11.3) and oscillations continued from the lower baseline. After the shift in the baseline the largest turbidity oscillations were recorded in this system. Subsequently, oscillations in turbidity and pH gradually ceased.
At 30 °C, turbidity was initially static at 32.5 until 1544 min from the start of the reaction at which point it dropped and began to fluctuate around a value of 30 (Fig. 1). At approximately 2500 min, the fluctuations in turbidity gradually became more regular. By 2800 min, regular sustained oscillations were established which lasted until the pH oscillations ceased at 3600 min (Fig. 9). Unlike at 20 °C, the oscillations in pH and turbidity were in phase.
At 40 °C no oscillatory behavior was apparent in turbidity although there was a sudden drop in turbidity from 32.5 at 2560 min, which settled at 31.3 by 2570 min. This drop coincided with the spike in pH and internal heater power that occurred when methanol was added to the reactor (Figs. 1 and 5a). The turbidity then fluctuated between 31 and 32 for the remainder of the reaction (Fig. 10). Regular oscillations in turbidity were not observed.
The oscillatory pH behavior in the PCPOC reaction system may be attributed to the oscillatory formation and consumption of HI which is initially formed by the reversible reaction given in Eq. 1 [15].
As previously shown, the occurrence of oscillations is accompanied by the increased rate of consumption of phenylacetylene [13], which can be described by Eq. 2 [14, 16]. This reaction appears to be autocatalytic in HI which accounts for the sharp drop in pH observed during each oscillation (Fig. 1).
Additionally, the consumption of HI is shown to be accounted for by Eq. 3, while the iodine produced subsequently reacts with the Pd species formed in Eq. 2, thereby regenerating the PdI2 catalyst (Eq. 4) [16].
Occasional samples taken during the reactions showed variations in the presence of a black precipitate that may be linked to the recorded turbidity behavior and, in particular, oscillations in turbidity via Eqs. 2 and 4. The depth of the color of the samples taken also varied. In the PCPOC reaction these color changes may be associated with changes in the concentration of iodine (Eqs. 3 and 4) or a soluble palladium complex (Eq. 1). As the turbidity probe may respond to changes in color and changes in particle formation both can be responsible for the observed trends.
Conclusions
The experiments described above confirm that the period and amplitude of pH oscillations in the PCPOC reaction increase as the reaction temperature decreases and the trends continue down to 0 °C. The duration of the oscillations also increases as the temperature decreases with sustained oscillations in batch being observed for more than 266 h (11 days) at 0 °C, the longest duration pH oscillations recorded in the PCPOC reaction, although the experiment was stopped before the oscillations had ceased. Sets of oscillations were observed at temperatures of 0 and 10 °C, whereas at 20–40 °C one main set of oscillations was observed. It was also found that temperatures of 0–30 °C produced changes in the shape of the waveform of the pH oscillations. Relaxation oscillations predominated when the oscillations were well established with oscillations having a more sinusoidal shape when the oscillations were very small (approx. 0.1 pH units). The addition of methanol to the reaction increased pH and amplitude of the oscillations. As methanol was already in excess this suggests water (present in trace amounts in the methanol) is involved in the oscillatory processes. Water has been reported to affect the pH behavior of the catalyst solution being involved in the reaction generating hydrogen ions [15]. Oscillations in turbidity were also observed. However, these were inconsistent between temperatures. At 0 °C large drops in turbidity occurred at the same time as the large final pH drop in each set of pH oscillations. At the same time regular turbidity oscillations were not observed. At 10 °C, following a large dip in turbidity which accompanied the initial pH drop, fluctuations in turbidity were recorded with no apparent oscillations present. At 20 °C the recorded turbidity oscillations were out of phase with the pH oscillations while at 30 °C the turbidity oscillations were in phase with the pH oscillations. At 40 °C, similarly to the 10 °C experiment, no clear oscillatory trend in turbidity was captured. The different behavior recorded at each temperature suggests an interplay between two processes, one involving iodine and another involving palladium (Eqs. 3 and 4). The very dark reaction mixture studied in these experiments makes it difficult to easily observe color changes or precipitate formation and therefore understand how each process relates to turbidity oscillations. Further studies experimenting at a reduced catalyst concentration would aid the investigation.
References
Gabriele B, Salerno G, Costa M (2006) Oxidative carbonylations. In: Beller M (ed) Catalytic carbonylation reactions, vol 18., Topics in organometallic chemistrySpringer, Berlin, pp 239–272
Gabriele B, Costa M, Salerno G, Chiusoli GP (1994) An efficient and selective palladium-catalysed oxidative dicarbonylation of alkynes to alkyl- or aryl-maleic esters. J Chem Soc Perkin Trans 1:83–87
Malashkevich AV, Bruk LG, Temkin ON (1997) New oscillating reaction in catalysis by metal complexes: a mechanism of alkyne oxidative carbonylation. J Phys Chem A 101(51):9825–9827
Bruk LG, Gorodskii SN, Zeigarnik AV, Valdés-Pérez RE, Temkin ON (1998) Oxidative carbonylation of phenylacetylene catalyzed by Pd(II) and Cu(I): experimental tests of forty-one computer-generated mechanistic hypotheses. J Mol Catal A 130(1–2):29–40
Bruk LG, Gorodski SN, Zeigarnik AV, Valdés-Peréz RE, Temkin ON (1999) Mechanism of oxidative carbonylation of phenylacetylene and methylacetylene. Generation and experimental discrimination of hypotheses. Russ Chem Bull 48(5):873–880
Gorodskii SN, Zakharov AN, Kulik AV, Bruk LG, Temkin ON (2001) Oxidative carbonylation of alkynes in an oscillation mode: I. Concentration limits for oscillations in the course of phenylacetylene carbonylation and possible mechanisms of the process. Kinet Catal 42(2):251–263
Gorodskii SN, Kalenova ES, Bruk LG, Temkin ON (2003) Oxidative carbonylation of alkynes in self-oscillating mode. Effect of the nature of substrates on the dynamic behavior of reaction system. Russ Chem Bull 52(7):1534–1543
Novakovic K, Grosjean C, Scott SK, Whiting A, Willis MJ, Wright AR (2007) Achieving pH and Qr oscillations in a palladium-catalysed phenylacetylene oxidative carbonylation reaction using an automated reactor system. Chem Phys Lett 435(1–3):142–147
Grosjean C, Novakovic K, Scott SK, Whiting A, Willis MJ, Wright AR (2008) Product identification and distribution from the oscillatory versus non-oscillatory palladium(II) iodide-catalysed oxidative carbonylation of phenylacetylene. J Mol Catal A 284(1–2):33–39
Parker J, Novakovic K (2013) Influence of water and the reactant addition sequence on palladium(II) iodide-catalyzed phenylacetylene carbonylation. Ind Eng Chem Res 52(7):2520–2527
Donlon L, Parker J, Novakovic K (2014) Oscillatory carbonylation of phenylacetylene in the absence of externally supplied oxidant. Reac Kinet Mech Cat 112(1):1–13
Novakovic K, Mukherjee A, Willis MJ, Wright AR, Scott SK (2009) The influence of reaction temperature on the oscillatory behaviour in the palladium-catalysed phenylacetylene oxidative carbonylation reaction. Phys Chem Chem Phys 11:9044–9049
Novakovic K, Grosjean C, Scott SK, Whiting A, Willis MJ, Wright AR (2008) The influence of oscillations on product selectivity during the palladium-catalysed phenylacetylene carbonylation reaction. Phys Chem Chem Phys 10:749–753
Donlon L, Novakovic K (2014) Oscillatory carbonylation using alkyne-functionalised poly(ethylene glycol). Chem Commun 50(98):15506–15508
Novakovic K, Parker J (2011) Catalyst initiation in the oscillatory carbonylation reaction. Int J Chem Eng
Gabriele B, Salerno G, Costa M, Chiusoli GP (2003) Recent developments in the synthesis of heterocyclic derivatives by PdI2-catalyzed oxidative carbonylation reactions. J Organomet Chem 687(2):219–228
Acknowledgments
The authors wish to acknowledge the UK Engineering and Physical Sciences Research Council (EPSRC) Grant no. EP/H003908/1 for funding this research. Mrs Julie Parker would like to acknowledge Professor Allen Wright for funding her Ph.D. studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Parker, J., Novakovic, K. Autonomous reorganization of the oscillatory phase in the PdI2 catalyzed phenylacetylene carbonylation reaction. Reac Kinet Mech Cat 118, 73–85 (2016). https://doi.org/10.1007/s11144-016-0979-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-0979-8