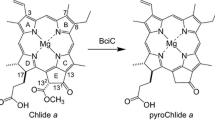
Abstract
Chlorophylls (Chls) are pigments involved in light capture and light reactions in photosynthesis. Chl a, Chl b, Chl d, and Chl f are characterized by unique absorbance maxima in the blue (Soret) and red (Qy) regions with Chl b, Chl d, and Chl f each possessing a single formyl group at a unique position. Relative to Chl a the Qy absorbance maximum of Chl b is blue-shifted while Chl d and Chl f are red-shifted with the shifts attributable to the relative positions of the formyl substitutions. Reduction of a formyl group of Chl b to form 7-hydroxymethyl Chl a, or oxidation of the vinyl group of Chl a into a formyl group to form Chl d was achieved using sodium borohydride (NaBH4) or β-mercaptoethanol (BME/O2), respectively. During the consecutive reactions of Chl b and Chl f using a three-step procedure (1. NaBH4, 2. BME/O2, and 3. NaBH4) two new 7-hydroxymethyl Chl a species were prepared possessing the 3-formyl or 3-hydroxymethyl groups and three new 2-hydroxymethyl Chl a species possessing the 3-vinyl, 3-formyl, or 3-hydroxymethyl groups, respectively. Identification of the spectral properties of 2-hydroxymethyl Chl a may be biologically significant for deducing the latter stages of Chl f biosynthesis if the mechanism parallels Chl b biosynthesis. The spectral features and chromatographic properties of these modified Chls are important for identifying potential intermediates in the biosynthesis of Chls such as Chl f and Chl d and for identification of any new Chls in nature.






Similar content being viewed by others
Abbreviations
- BME:
-
β-Mercaptoethanol
- CAO:
-
Chlorophyll a oxygenase
- CBR:
-
Chlorophyll b reductase
- Chl:
-
Chlorophyll
- Chlide:
-
Chlorophyllide
- HCAR:
-
Hydroxymethyl-chlorophyll a reductase
- MALDI-MS:
-
Matrix-assisted laser desorption/ionization mass spectrometry
- NaBH4 :
-
Sodium borohydride
- RP-HPLC:
-
Reversed-phase high-performance liquid chromatography
References
Björn LO, Papageorgiou GC, Blankenship RE, Govindjee (2009) A viewpoint: why chlorophyll a? Photosynth Res 99(2):85–98. https://doi.org/10.1007/s11120-008-9395-x
Borch RF, Bernstein MD, Durst HD (1971) Cyanohydridoborate anion as a selective reducing agent. J Am Chem Soc 93(12):2897–2904. https://doi.org/10.1021/ja00741a013
Chaikin SW, Brown WG (1949) Reduction of aldehydes, ketones and acid chlorides by sodium borohydride. J Am Chem Soc 71(1):122–125. https://doi.org/10.1021/ja01169a033
Chen M (2014) Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu Rev Biochem 83(1):317–340. https://doi.org/10.1146/annurev-biochem-072711-162943 doi
Chen M, Schliep M, Willows RD, Cai Z-L, Neilan BA, Scheer H (2010) A red-shifted chlorophyll. Science 329(5997):1318–1319. https://doi.org/10.1126/science.1191127
Chen M, Li Y, Birch D, Willows RD (2012) A cyanobacterium that contains chlorophyll f—a red-absorbing photopigment. FEBS Lett 586(19):3249–3254. https://doi.org/10.1016/j.febslet.2012.06.045
Eggink LL, Park H, Hoober JK (2001) The role of chlorophyll b in photosynthesis: hypothesis. BMC Plant Biol 1(1):2. https://doi.org/10.1186/1471-2229-1-2
Eggink LL, LoBrutto R, Brune DC, Brusslan J, Yamasato A, Tanaka A, Hoober JK (2004) Synthesis of chlorophyll b: localization of chlorophyllide a oxygenase and discovery of a stable radical in the catalytic subunit. BMC Plant Biol 4:5. https://doi.org/10.1186/1471-2229-4-5
Fukusumi T, Matsuda K, Mizoguchi T, Miyatake T, Ito S, Ikeda T, Tamiaki H, Oba T (2012) Non-enzymatic conversion of chlorophyll-a into chlorophyll-d in vitro: a model oxidation pathway for chlorophyll-d biosynthesis. FEBS Lett 586(16):2338–2341. https://doi.org/10.1016/j.febslet.2012.05.036
Garg H, Loughlin PC, Willows RD, Chen M (2017) The C21-formyl group in chlorophyll f originates from molecular oxygen. J Biol Chem 292(47):19279–19289. https://doi.org/10.1074/jbc.M117.814756
Gouterman M, Wagnière GH, Snyder LC (1963) Spectra of porphyrins: Part II. Four orbital model. J Mol Spectrosc 11(1):108–127. https://doi.org/10.1016/0022-2852(63)90011-0
Ho M-Y, Shen G, Canniffe DP, Zhao C, Bryant DA (2016) Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of photosystem II. Science 353 (6302). https://doi.org/10.1126/science.aaf9178
Hohmann-Marriott MF, Blankenship RE (2011) Evolution of photosynthesis. Annu Rev Plant Biol 62(1):515–548. https://doi.org/10.1146/annurev-arplant-042110-103811
Holt AS (1959) Reduction of chlorophyllides, chlorophylls and chlorophyll derivatives by sodium borohydride. Plant Physiol 34(3):310–314
Holt AS, Morley HV (1959) A proposed structure for chlorophyll d. Can J Chem 37(3):507–514. https://doi.org/10.1139/v59-069
Hoober JK, Eggink LL, Chen M (2007) Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth Res 94(2):387–400. https://doi.org/10.1007/s11120-007-9181-1
Hu Q, Miyashita H, Iwasaki I, Kurano N, Miyachi S, Iwaki M, Itoh S (1998) A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis. Proc Natl Acad Sci USA 95(22):13319–13323. https://doi.org/10.1073/pnas.95.22.13319
Hynninen PH, Kaartinen V, Kolehmainen E (2010) Horseradish peroxidase-catalyzed oxidation of chlorophyll a with hydrogen peroxide: characterization of the products and mechanism of the reaction. Biochim Biophys Acta 1797(5):531–542. https://doi.org/10.1016/j.bbabio.2010.01.017
Hynninen Paavo H, Sievers G (1981) Conformations of chlorophylls a and a′ and their magnesium-free derivatives as revealed by circular dichroism and proton magnetic resonance. znb 36 (8):1000–1009. https://doi.org/10.1515/znb-1981-0819
Hyvärinen K, Helaja J, Kuronen P, Kilpeläinen I, Hynninen PH (1995)) 1H and 13C NMR spectra of the methanolic allomerization products of 132(R)-chlorophyll a. Magn Reson Chem 33(8):646–656. https://doi.org/10.1002/mrc.1260330806 doi
Ito H, Ohtsuka T, Tanaka A (1996) Conversion of chlorophyll b to chlorophyll a via 7-hydroxymethyl chlorophyll. J Biol Chem 271(3):1475–1479. https://doi.org/10.1074/jbc.271.3.1475
Keller MD, Selvin RC, Claus W, Guillard RRL (1987) Media for the culture of oceanic ultraphytoplankton. J Phycol 23(4):633–638. https://doi.org/10.1111/j.1529-8817.1987.tb04217.x doi
Koizumi H, Itoh Y, Hosoda S, Akiyama M, Hoshino T, Shiraiwa Y, Kobayashi M (2005) Serendipitous discovery of Chl d formation from Chl a with papain. Sci Technol Adv Mater 6(6):551–557. https://doi.org/10.1016/j.stam.2005.06.022
Kräutler B (2011) A new factor in life’s quest for energy. Angew Chem Int Ed Engl 50(11):2439–2441. https://doi.org/10.1002/anie.201007339 doi
Kume A, Akitsu T, Nasahara KN (2018) Why is chlorophyll b only used in light-harvesting systems? J Plant Res. https://doi.org/10.1007/s10265-018-1052-7
Kusunoki M, Tamiaki H (2018) Synthesis of 7-substituted chlorophyll-a derivatives as chlorophyll-b analogs with specific visible absorption bands. Tetrahedron 74(4):453–464. https://doi.org/10.1016/j.tet.2017.12.013
Lane CF (1975) Sodium cyanoborohydride—a highly selective reducing agent for organic functional groups. Synthesis 1975 (03):135–146. https://doi.org/10.1055/s-1975-23685
Li Y, Lin Y, Loughlin P, Chen M (2014) Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris—a filamentous cyanobacterium containing chlorophyll f. Front Plant Sci 5 (67). https://doi.org/10.3389/fpls.2014.00067
Li Y, Vella N, Chen M (2018) Characterization of isolated photosystem I from Halomicronema hongdechloris, a chlorophyll f-producing cyanobacterium. Photosynthetica 56(1):306–315. https://doi.org/10.1007/s11099-018-0776-x
Loughlin PC, Willows RD, Chen M (2014) In vitro conversion of vinyl to formyl groups in naturally occurring chlorophylls. Sci Rep 4:6069. https://doi.org/10.1038/srep06069
Loughlin PC, Willows RD, Min C (2015) Hydroxylation of the C132 and C18 carbons of chlorophylls by heme and molecular oxygen. J Porphyr Phthalocyanines 19(09):1007–1013. https://doi.org/10.1142/s1088424615500571
Manning WM, Strain HH (1943) Chlorophyll d, a green pigment of red algae. J Biol Chem 151(1):1–19
Meguro M, Ito H, Takabayashi A, Tanaka R, Tanaka A (2011) Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. Plant Cell 23(9):3442–3453. https://doi.org/10.1105/tpc.111.089714
Miyashita H, Ikemoto H, Kurano N, Adachi K, Chihara M, Miyachi S (1996) Chlorophyll d as a major pigment. Nature 383:402. https://doi.org/10.1038/383402a0
Nürnberg DJ, Morton J, Santabarbara S, Telfer A, Joliot P, Antonaru LA, Ruban AV, Cardona T, Krausz E, Boussac A, Fantuzzi A, Rutherford AW (2018) Photochemistry beyond the red limit in chlorophyll f-containing photosystems. Science 360(6394):1210–1213. https://doi.org/10.1126/science.aar8313
Oba T, Uda Y, Matsuda K, Fukusumi T, Ito S, Hiratani K, Tamiaki H (2011) A mild conversion from 3-vinyl- to 3-formyl-chlorophyll derivatives. Bioorg Med Chem Lett 21(8):2489–2491. https://doi.org/10.1016/j.bmcl.2011.02.054
Oster U, Tanaka R, Tanaka A, Rüdiger W (2000) Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J 21(3):305–310. https://doi.org/10.1046/j.1365-313x.2000.00672.x doi
Partensky F, Six C, Ratin M, Garczarek L, Vaulot D, Probert I, Calteau A, Gourvil P, Marie D, Grébert T, Bouchier C, Le Panse S, Gachenot M, Rodríguez F, Garrido JL (2018) A novel species of the marine cyanobacterium Acaryochloris with a unique pigment content and lifestyle. Sci Rep 8(1):9142. https://doi.org/10.1038/s41598-018-27542-7
Reinbothe S, Pollmann S, Reinbothe C (2003) In situ conversion of protochlorophyllide b to protochlorophyllide a in barley: evidence for a novel role of 7-formyl reductase in the prolamellar body of etioplasts. J Biol Chem 278(2):800–806. https://doi.org/10.1074/jbc.M209737200
Scheumann V, Schoch S, Rüdiger W (1998) Chlorophyll a formation in the chlorophyll b reductase reaction requires reduced ferredoxin. J Biol Chem 273(52):35102–35108. https://doi.org/10.1074/jbc.273.52.35102
Schliep M, Crossett B, Willows RD, Chen M (2010)) 18O labeling of chlorophyll d in Acaryochloris marina reveals that chlorophyll a and molecular oxygen are precursors. J Biol Chem 285(37):28450–28456. https://doi.org/10.1074/jbc.M110.146753
Schmitt F-J, Campbell ZY, Bui MV, Hüls A, Tomo T, Chen M, Maksimov EG, Allakhverdiev SI, Friedrich T (2018) Photosynthesis supported by a chlorophyll f-dependent, entropy-driven uphill energy transfer in Halomicronema hongdechloris cells adapted to far-red light. Photosynth Res. https://doi.org/10.1007/s11120-018-0556-2
Strain HH, Manning WM (1942) Isomerization of chlorophylls a and b. J Biol Chem 146(1):275–276
Tamiaki H, Miyata S, Kureishi Y, Tanikaga R (1996) Aggregation of synthetic zinc chlorins with several esterified alkyl chains as models of bacteriochlorophyll-c homologs. Tetrahedron 52(38):12421–12432. https://doi.org/10.1016/0040-4020(96)00740-5
Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci 95(21):12719–12723
Tomo T, Suzuki T, Hirano E, Tsuchiya T, Miyashita H, Dohmae N, Mimuro M (2006) Reversible absorption change of chlorophyll d in solutions. Chem Phys Lett 423(4):282–287. https://doi.org/10.1016/j.cplett.2006.03.091
Tsuchiya T, Mizoguchi T, Akimoto S, Tomo T, Tamiaki H, Mimuro M (2012) Metabolic engineering of the Chl d-dominated cyanobacterium Acaryochloris marina: production of a novel Chl species by the introduction of the chlorophyllide a oxygenase gene. Plant Cell Physiol 53(3):518–527. https://doi.org/10.1093/pcp/pcs007
Voitsekhovskaja OV, Tyutereva EV (2015) Chlorophyll b in angiosperms: functions in photosynthesis, signaling and ontogenetic regulation. J Plant Physiol 189:51–64. https://doi.org/10.1016/j.jplph.2015.09.013
Xu M, Kinoshita Y, Tamiaki H (2014) Synthesis of chlorophyll-f analogs possessing the 2-formyl group by modifying chlorophyll-a. Bioorg Med Chem Lett 24(16):3997–4000. https://doi.org/10.1016/j.bmcl.2014.06.022
Acknowledgements
We would like to thank Dr. Ben Crossett for access to the mass spectrometry facility at the Charles Perkins Centre, The University of Sydney. We also thank Dr. Ann Kwan for assisting with the NMR experiments.
Funding
The research is supported by The Australian Research Council Centre of Excellence for Translational Photosynthesis (CE20140100015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sawicki, A., Willows, R.D. & Chen, M. Spectral signatures of five hydroxymethyl chlorophyll a derivatives chemically derived from chlorophyll b or chlorophyll f. Photosynth Res 140, 115–127 (2019). https://doi.org/10.1007/s11120-018-00611-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-00611-8