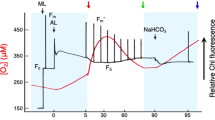
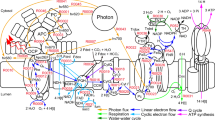
Abstract
Some cyanobacteria, but not all, experience an induction of alternative electron flow (AEF) during CO2-limited photosynthesis. For example, Synechocystis sp. PCC 6803 (S. 6803) exhibits AEF, but Synechococcus elongatus sp. PCC 7942 does not. This difference is due to the presence of flavodiiron 2 and 4 proteins (FLV2/4) in S. 6803, which catalyze electron donation to O2. In this study, we observed a low-[CO2] induced AEF in the marine cyanobacterium Synechococcus sp. PCC 7002 that lacks FLV2/4. The AEF shows high affinity for O2, compared with AEF mediated by FLV2/4 in S. 6803, and can proceed under extreme low [O2] (about a few µM O2). Further, the transition from CO2-saturated to CO2-limited photosynthesis leads a preferential excitation of PSI to PSII and increased non-photochemical quenching of chlorophyll fluorescence. We found that the model green alga Chlamydomonas reinhardtii also has an O2-dependent AEF showing the same affinity for O2 as that in S. 7002. These data represent the diverse molecular mechanisms to drive AEF in cyanobacteria and green algae. In this paper, we further discuss the diversity, the evolution, and the physiological function of strategy to CO2-limitation in cyanobacterial and green algal photosynthesis.






Similar content being viewed by others
Abbreviations
- AEF:
-
Alternative electron flow
- FLV:
-
Flavodiiron protein
- PET:
-
Photosynthetic electron transport
- NPQ:
-
Non-photochemical quenching
References
Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro EM (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110:4111–4116
Appel J, Phunpruch S, Steinmüller K, Schulz R (2000) The bidirectional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Microbiology 173:333–378
Arnon DI, Allen MB, Whatley FR (1954) Photosynthesis by isolated chloroplasts. Nature 1174:394396
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Beardall J, Quigg A, Raven JA (2003) Oxygen consumption: photorespiration and chlororespiration. In: Larkum WD, Douglas SE, Raven JA (eds) Photosynthesis in algae. Kluwer Academic Publishers, Dordrecht, pp 157–181
Bernát G, Rögner M (2011) Center of the cyanobacterial electron transport network: the cytochrome b 6 f complex. In: Peschek G, Obinger C, Renger G (eds) Bioenergetic processes of cyanobacteria. Springer, Dordrecht, pp 573–606
Birmingham BC, Coleman JR, Colman B (1982) Measurement of photorespiration in algae. Plant Physiol 69:259–262
Campbell D, Hurry V, Clarke AK, Gustafsson P, Öquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62:667–683
Demmig-Adams B, Garab G, Adams III, Govindjee W (eds) (2014) Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria. Springer, Dordrecht
Dvořák P, Casamatta DA, Poulíčková A, Hašler P, Ondřej V, Sanges R (2014) Synechococcus: 3 billion years of global dominance. Mol Ecol 23:5538–5551
Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M (2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc Natl Acad Sci USA 105:17199–17204
Falkowski PG, Fujita Y, Ley A, Mauzerall D (1986) Evidence for cyclic electron flow around photosystem II in Chlorella pyrenoidosa. Plant Physiol 81:310–312
Flores E, Frías JE, Rubio LM, Herrero A (2005) Photosynthetic nitrate assimilation in cyanobacteria. Photosynth Res 83:117–133
Fujimori T, Hihara Y, Sonoike K (2005) PsaK2 subunit in photosystem I is involved in state transition under high light condition in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 280:22191–22197
Fujisawa T, Okamoto S, Katayama T, Nakao M, Yoshimura H, Kajiya-Kanegae H, Yamamoto S, Yano C, Yanaka Y, Maita H, Kaneko T, Tabata S, Nakamura Y (2014) CyanoBase and RhizoBase: databases of manually curated annotations for cyanobacterial and rhizobial genomes. Nucl Acids Res 42:666–670
Govindjee Björn LO (2015) Dissecting oxygenic photosynthesis: The evolution of the “Z”-scheme for thylakoid reactions. In: Itoh S, Mohanty P, Guruprasad KN (eds) Photosynthesis: overviews on recent progress and future perspective. Publishers, New Delhi, I.K, pp 1–27
Govindjee Amesz J, Fork DC (eds) (1986) Light emission by plants and bacteria. Academic Press, NY
Grimme LH, Boardman NK (1972) Photochemical activities of a particle fraction P1 obtained from the green alga Chlorella fuska. Biochem Biophys Res Commun 49:1617–1623
Hayashi R, Shimakawa G, Shaku K, Shimizu S, Akimoto S, Yamamoto H, Amako K, Sugimoto T, Tamoi M, Makino A, Miyake C (2014) O2-dependent large electron flow functioned as an electron sink, replacing the steady-state electron flux in photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803, but not in the cyanobacterium Synechococcus sp. PCC 7942. Biosci Biotechnol Biochem 78:384–393
Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I, Kaplan A (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13:230–235
Helman Y, Barkan E, Eisenstadt D, Luz B, Kaplan A (2005) Fractionation of the three stable oxygen isotopes by oxygen-producing and oxygen-consuming reactions in photosynthetic organisms. Plant Physiol 138:2292–2298
Hihara Y, Kamei A, Kanehisa M, Kaplan A, Ikeuchi M (2001) DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13:793–806
Ihnken S, Kromkamp JC, Beardall J, Silsbe GM (2013) State-transitions facilitate robust quantum yields and cause an over-estimation of electron transport in Dunaliella tertiolecta cells held at the CO2 compensation point and re-supplied with DIC. Photosynth Res 119:257–272
Iwai M, Kato N, Minagawa J (2007) Distinct physiological responses to a high light and low CO2 environment revealed by fluorescence quenching in photoautotrophically grown Chlamydomonas reinhardtii. Photosynth Res 94:307–314
Jordan DB, Ogren WL (1981) Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature 291:513–515
Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192:261–268
Klughammer C, Schreiber U (2008) Saturation pulse method for assessment of energy conversion in PSI. PAM Appl Notes 1:11–14
Kodru S, Malavath T, Devadasu E, Nellaepalli S, Stirbet A, Subramanyam R, Govindjee (2015) The slow S to M rise of chlorophyll a fluorescence reflects transition from state 2 to state 1 in the green alga Chlamydomonas reinhardtii. Photosynth Res 125:219–231
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56:337–346
Kusama Y, Inoue S, Jimbo H, Takaichi S, Sonoike K, Hihara Y, Nishiyama Y (2015) Zeaxanthin and echinenone protect the repair of photosystem II from inhibition by singlet oxygen in Synechocystis sp. PCC 6803. Plant Cell Physiol 56:906–916
Ley AC, Butler WL (1980) Energy distribution in the photochemical apparatus of Porphyridium cruentum in state I and state II. Biochim Biophys Acta 592:349–363
Lichtenthaler HK, Buchanan BB, Douce R, Govindjee (2015) Andrew A. Benson, 1917–2015. Photosynth Res 124:131–135
Marek LF, Spalding MH (1991) Changes in photorespiratory enzyme activity in response to limiting CO2 in Chlamydomonas reinhardtii. Plant Physiol 97:420–425
McConnell MD, Koop R, Vasil’ev S, Bruce S (2002) Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria. A structure-based model for the light state transition. Plant Physiol 130:1201–1212
Mehler AH (1951) Studies on reactivities of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys 33:65–77
Miller AG, Espie GS, Bruce D (1996) Characterization of the non-photochemical quenching of chlorophyll fluorescence that occurs during the active accumulation of inorganic carbon in the cyanobacterium Synechococcus PCC 7942. Photosynth Res 49:251–262
Miyake C (2010) Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963
Miyake C, Asada K (2003) The water-water cycle in algae. In: Larkum WD, Douglas SE, Raven JA (eds) Photosynthesis in algae. Kluwer Academic Publishers, Dordrecht, pp 183–204
Miyake C, Yokota A (2001) Cyclic flow of electrons within PSII in thylakoid membranes. Plant Cell Physiol 42:508–515
Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves—relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46:629–637
Moroney JV, Wilson BJ, Tolbert NE (1986) Glycolate metabolism and excretion by Chlamydomonas reinhardtii. Plant Physiol 82:821–826
Murata N (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172:242–251
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998
Najafpour M, Moghaddam AN, Shen J-R, Govindjee (2013) Water oxidation and water oxidizing complex in cyanobacteria. In: Srivastava A et al (eds) Stress biology of cyanobacteria. Taylor & Francis, UK, pp 41–60
Niyogi KK, Truong TB (2013) Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 16:307–314
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in Photosynthesis and Respiration, Springer, Dordrecht
Papageorgiou GC, Govindjee (2011) Photosystem II fluorescence: slow changes-Scaling from the past. J Photochem Photobiol B 104:258–270
Papageorgiou GC, Tsimilli-Michael M, Stamatakis K (2007) The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint. Photosynth Res 94:275–290
Roach T, Na CS, Krieger-Liszkay A (2015) High light-induced hydrogen peroxide production in Chlamydomonas reinhardtii is increased by high CO2 availability. Plant J 81:759–766
Satoh S, Mimuro M, Tanaka A (2013) Construction of a phylogenetic tree of photosynthetic prokaryotes based on average similarities of whole genome sequences. PLoS One 8:e70290
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Schuurmans RM, van Alphen P, Schuurmans JM, Matthijs HCP, Hellingwerf KJ (2015) Comparison of the photosynthetic yield of cyanobacteria and green algae: different methods give different answers. PLoS One 10:e0139061
Sejima T, Takagi D, Fukayama H, Makino A, Miyake C (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol 55:1184–1193
Sejima T, Hanawa H, Shimakawa G, Takagi D, Suzuki Y, Fukayama H, Makino A, Miyake C (2015) Post-illumination transient O2-uptake is driven by photorespiration in tobacco leaves. Physiol Plant 156:227–238
Shaku K, Shimakawa G, Hashiguchi M, Miyake C (2015) Reduction-induced suppression of electron flow (RISE) in the photosynthetic electron transport system of Synechococcus elongatus PCC 7942. Plant Cell Physiol. doi:10.1093/pcp/pcv198
Shikanai T (2016) Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I. Photosynth Res. doi:10.1007/s11120-016-0227-0
Shimakawa G, Shaku K, Nishi A, Hayashi R, Yamamoto H, Sakamoto K, Makino A, Miyake C (2015) FLAVODIIRON2 and FLAVODIIRON4 proteins mediate and oxygen-dependent alternative electron flow in Synechocystis sp. PCC 6803 under CO2-limited conditions. Plant Physiol 167:472–480
Stevens SE, Porter RD (1980) Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci USA 77:6052–6056
Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Planta 228:1055–1066
Sültemeyer DF, Miller AG, Espie GS, Fock HP, Canvin DT (1989) Active CO2 transport by the green alga Chlamydomonas reinhardtii. Plant Physiol 89:1213–1219
Turner S, Pryer KM, Miao VPW, Palmer JD (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46:327–338
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
van Thor JJ, Mullineaux CW, Matthijs HCP, Hellingwerf KJ (1998) Light harvesting and state transitions in cyanobacteria. Bot Acta 111:430–443
Vicente JB, Gomes CM, Wasserfallen A, Teixeira M (2002) Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem Biophys Res Commun 294:82–87
Wilson A, Ajlani G, Verbavatz JM, Vass I, Kerfeld CA, Kirilovsky D (2006) A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18:992–1007
Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM (2009) Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4:e5331
Zhou J-J, Fernández E, Galván A, Miller AJ (2000) A high affnity nitrate transport system from Chlamydomonas requires two gene products. FEBS Lett 466:225–227
Zivcak M, Brestic M, Kunderlikova K, Sytar O, Allakhverdiev SI (2015a) Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth Res 126:449–463
Zivcak M, Brestic M, Kunderlikova K, Olsovska K, Allakhverdiev SI (2015b) Effect of photosystem I inactivation on chlorophyll a fluorescence induction in wheat leaves: does activity of photosystem I play any role in OJIP rise? J Photochem Photobiol B 152:318–324
Acknowledgments
The authors thank Prof. Akihiko Kondo, Prof. Tomohisa Hasunuma, and Dr. Shimpei Aikawa for the supply of the Synechococcus sp. PCC 7002 wild type.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shimakawa, G., Akimoto, S., Ueno, Y. et al. Diversity in photosynthetic electron transport under [CO2]-limitation: the cyanobacterium Synechococcus sp. PCC 7002 and green alga Chlamydomonas reinhardtii drive an O2-dependent alternative electron flow and non-photochemical quenching of chlorophyll fluorescence during CO2-limited photosynthesis. Photosynth Res 130, 293–305 (2016). https://doi.org/10.1007/s11120-016-0253-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0253-y